| Phenethyl alcohol |
| Phenylethyl alcohol |
| 60-12-8 |
| Benzeneethanol |
| Phenylethanol |
| Benzyl carbinol |
| Phenethanol |
| 2-Phenylethyl alcohol |
| 2-PHENYL-ETHANOL |
| beta-Phenylethanol |
| 2-Phenethyl alcohol |
| Benzylmethanol |
| 2-Phenylethan-1-Ol |
| Benzylcarbinol |
| Methanol, benzyl- |
| 2-Hydroxyethylbenzene |
| 1-Phenyl-2-ethanol |
| Ethanol, 2-phenyl- |
| FEMA No. 2858 |
| 2-PEA |
| Benzenethanol |
| Phenethylalcohol |
| Phenyl ethyl alcohol |
| beta-PEA |
| beta-Phenylethyl alcohol |
| 1321-27-3 |
| beta-Hydroxyethylbenzene |
| Caswell No. 655C |
| beta-Fenylethanol |
| FEMA Number 2858 |
| Ethanol, phenyl- |
| beta-Fenethylalkohol |
| Phenethyl alcohol (natural) |
| beta-Phenethyl alcohol |
| HSDB 5002 |
| 2-Phenethanol |
| .beta.-Hydroxyethylbenzene |
| beta-Fenylethanol [Czech] |
| .beta.-Phenylethyl alcohol |
| Hydroxyethylbenzene |
| EINECS 200-456-2 |
| UNII-ML9LGA7468 |
| MFCD00002886 |
| beta-Fenethylalkohol [Czech] |
| PhenethylAlcohol-d5 |
| EPA Pesticide Chemical Code 001503 |
| NSC 406252 |
| NSC-406252 |
| BRN 1905732 |
| .beta.-Phenylethanol |
| ML9LGA7468 |
| .beta.-PEA |
| DTXSID9026342 |
| CHEBI:49000 |
| AI3-00744 |
| (2-Hydroxyethyl)benzene |
| .beta.-Phenethyl alcohol |
| Phenylethyl alcohol [USP] |
| .beta.-(hydroxyethyl)benzene |
| DTXCID206342 |
| EC 200-456-2 |
| 4-06-00-03067 (Beilstein Handbook Reference) |
| NSC406252 |
| NCGC00166215-02 |
| Phenylethyl alcohol (USP) |
| PHENYLETHYL ALCOHOL (II) |
| PHENYLETHYL ALCOHOL [II] |
| PHENETHYL ALCOHOL (MART.) |
| PHENETHYL ALCOHOL [MART.] |
| Phenyl Ethanol(Natural) |
| 2 Phenylethanol |
| PHENYLETHYL ALCOHOL (USP-RS) |
| PHENYLETHYL ALCOHOL [USP-RS] |
| 2-phenyl ethanol |
| Carbinol, Benzyl |
| beta Phenylethanol |
| CAS-60-12-8 |
| Alcohol, Phenethyl |
| PEL |
| SMR000059156 |
| PHENYLETHYL ALCOHOL (USP MONOGRAPH) |
| PHENYLETHYL ALCOHOL [USP MONOGRAPH] |
| Alcohol, Phenylethyl |
| Bencenoetanol |
| benzene-ethanol |
| Mellol |
| phenyl-ethanol |
| Benzeneethanol- |
| Benzyl-Methanol |
| 2-PhenyIethanol |
| 2- phenylethanol |
| phenylethyl-alcohol |
| .beta.-Phenethanol |
| 2 - phenylethanol |
| HY1 |
| .beta.-Fenylethanol |
| b-Hydroxyethylbenzene |
| Benzyl ethyl alcohol |
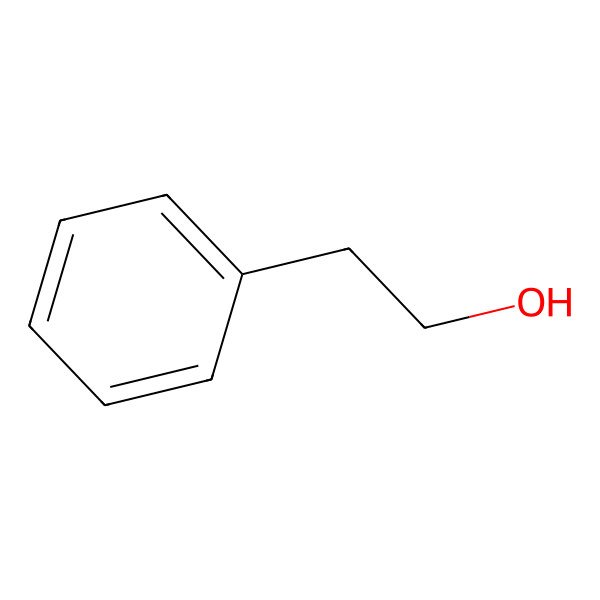
| C(CO)c1ccccc1 |
| 2-phenyl-1-ethanol |
| Benzeneethanol, 9CI |
| 2-phenylethane-1-ol |
| betaphenylethyl alcohol |
| .beta.-Fenethylalkohol |
| 2-Phenylethanol, USP |
| METHANOL, BENZYL |
| A-PEA |
| beta -hydroxyethylbenzene |
| 2-Phenylethanol, 99% |
| .beta.-P.E.A. |
| (BETA-PEA) |
| Phenylethyl alcohol, USAN |
| bmse000659 |
| Phenylethyl, beta- alcohol |
| 2-(2-Hydroxyethyl)benzene |
| SCHEMBL1838 |
| WLN: Q2R |
| MLS001066349 |
| MLS001336026 |
| FEMA NUMBER 2858. |
| PHENETHYL ALCOHOL [MI] |
| Phenethyl alcohol, 8CI, BAN |
| CHEMBL448500 |
| beta-(HYDROXYETHYL)BENZENE |
| PHENETHYL ALCOHOL [FCC] |
| PHENYLETHYL, B- ALCOHOL |
| PHENETHYL ALCOHOL [INCI] |
| BDBM85807 |
| FEMA 2858 |
| HMS2093H05 |
| HMS2233H06 |
| HMS3374P04 |
| Pharmakon1600-01505398 |
| PHENYLETHYL ALCOHOL [FHFI] |
| PHENYLETHYL ALCOHOL [HSDB] |
| PHENETHYL ALCOHOL [WHO-DD] |
| BCP32115 |
| CS-B1821 |
| HY-B1290 |
| NSC_6054 |
| Tox21_113544 |
| Tox21_201322 |
| Tox21_303383 |
| BBL036905 |
| NSC759116 |
| s3703 |
| STL281950 |
| 2-Phenylethanol, >=99.0% (GC) |
| AKOS000249688 |
| Tox21_113544_1 |
| CCG-213419 |
| DB02192 |
| LS-2335 |
| NSC-759116 |
| CAS_60-12-8 |
| Phenethyl alcohol, >=99%, FCC, FG |
| NCGC00166215-01 |
| NCGC00166215-03 |
| NCGC00166215-05 |
| NCGC00257347-01 |
| NCGC00258874-01 |
| AC-18484 |
| SBI-0206858.P001 |
| FT-0613332 |
| FT-0673679 |
| P0084 |
| EN300-19347 |
| C05853 |
| D00192 |
| D70868 |
| Phenethyl alcohol, natural, >=99%, FCC, FG |
| AB00698274_05 |
| A832606 |
| Q209463 |
| SR-01000763553 |
| Phenylethyl alcohol;Phenethyl alcohol;Benzeneethanol |
| Q-200318 |
| SR-01000763553-2 |
| 0DE4CADC-AB8A-4038-BD6F-EBD009885652 |
| F0001-1575 |
| Z104473586 |
| 2-phenylethanol;2-Phenylethyl alcohol;Benzeneethanol;Phenylethanol |
| InChI=1/C8H10O/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H |
| Phenylethyl alcohol, United States Pharmacopeia (USP) Reference Standard |
| Phenylethyl Alcohol, Pharmaceutical Secondary Standard; Certified Reference Material |
| 19601-20-8 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|