| 29106-49-8 |
| Procyanidol B2 |
| Proanthocyanidin B2 |
| (+)-Procyanidin B2 |
| Procyanidin B-2 |
| CHEBI:75632 |
| 2,3-cis-proanthocyanidin |
| UNII-L88HKE854X |
| L88HKE854X |
| EC-(4b,8)-EC |
| Epicathechin-(4beta->8)-epicathechin |
| NSC 623097 |
| PROCYANIDIN B2 DIMER |
| CHEMBL38714 |
| PROCYANIDIN B2, (+)- |
| NSC-623097 |
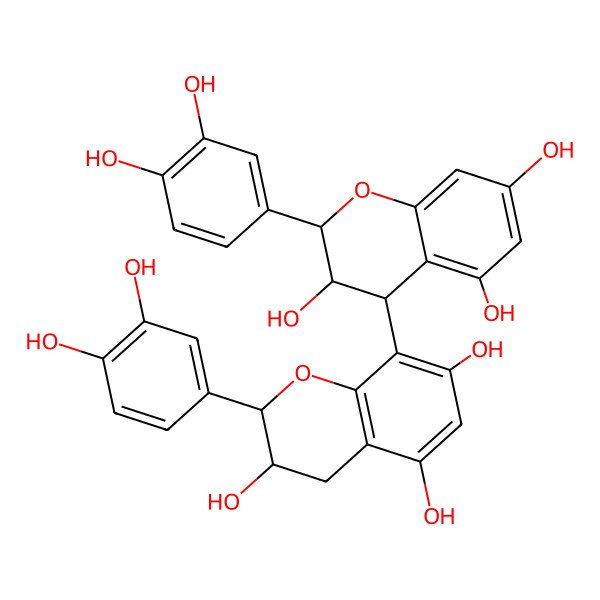
| (2R,3R)-2-(3,4-dihydroxyphenyl)-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol |
| [4,8'-Bi-2H-1-benzopyran]-3,3',5,5',7,7'-hexol, 2,2'-bis(3,4-dihydroxyphenyl)-3,3',4,4'-tetrahydro-, (2R,2'R,3R,3'R,4R)- |
| (2R-(2alpha,3alpha,4beta(2'R*,3'R*)))-2,2'-Bis(3,4-dihydroxyphenyl)-3,3',4,4'-tetrahydro-(4,8'-Bi-2H-1-benzopyran)-3,3',5,5',7,7'-hexol |
| (4,8'-Bi-2H-1-benzopyran)-3,3',5,5',7,7'-hexol, 2,2'-bis(3,4-dihydroxyphenyl)-3,3',4,4'-tetrahydro-, (2R-(2alpha,3alpha,4beta(2'R*,3'R*)))- |
| PROCYANIDIN B2 (USP-RS) |
| PROCYANIDIN B2 [USP-RS] |
| (2R,2'R,3R,3'R,4R)-2,2'-bis(3,4-dihydroxyphenyl)-3,3',4,4'-tetrahydro-2H,2'H-4,8'-bichromene-3,3',5,5',7,7'-hexol |
| (4,8'-BI-2H-1-BENZOPYRAN)-3,3',5,5',7,7'-HEXOL, 2,2'-BIS(3,4-DIHYDROXYPHENYL)-3,3',4,4'-TETRAHYDRO-, (2R,2'R,3R,3'R,4R)- |
| PROCYANIDINB2 |
| Procyanidin-B2 |
| C30-H26-O12 |
| Procyanidin dimer B2 |
| SCHEMBL288579 |
| XFZJEEAOWLFHDH-NFJBMHMQSA-N |
| DTXSID701028797 |
| HY-N0796 |
| Procyanidin B2, analytical standard |
| BDBM50553253 |
| LMPK12030002 |
| MFCD01861513 |
| AKOS008901339 |
| CS-5982 |
| BS-49221 |
| 4,8″-Bi-[(+)-epicatechin], cis |
| C17639 |
| (-)-Epicatechin-(4.beta.-8)-(-)-epicatechin |
| A912693 |
| J-017393 |
| Q7247552 |
| (-)-EPICATECHIN-(4beta->8)-(-)-EPICATECHIN |
| (-)-EPICATECHIN-(4.BETA.->8)-(-)-EPICATECHIN |
| cis,cis"-4,8"-Bi(3,3',4',5,7-pentahydroxyflavane) |
| cis″-4,8″-Bi(3,3',4',5,7-pentahydroxyflavane) |
| PROCYANIDIN B2 (CONSTITUENT OF GRAPE SEEDS OLIGOMERIC PROANTHOCYANIDINS) |
| (2R,2'R,3R,3'R,4R)-2,2'-Bis(3,4-dihydroxyphenyl)-[4,8'-bichromane]-3,3',5,5',7,7'-hexaol |
| (2R,2'R,3R,3'R,4R)-2,2'-Bis(3,4-dihydroxyphenyl)-4,8'-bichroman-3,3',5,5',7,7'-hexol |
| (2R,3R,4R)-2-(3,4-dihydroxyphenyl)-4-[(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chroman-8-yl]chromane-3,5,7-triol |
| (2R-(2.ALPHA.,3.ALPHA.,4.BETA.(2'R*,3'R*)))-2,2'-BIS(3,4-DIHYDROXYPHENYL)-3,3',4,4'-TETRAHYDRO-(4,8'-BI-2H-1-BENZOPYRAN)-3,3',5,5',7,7'-HEXOL |
| (4,8'-BI-2H-1-BENZOPYRAN)-3,3',5,5',7,7'-HEXOL, 2,2'-BIS(3,4-DIHYDROXYPHENYL)-3,3',4,4'-TETRAHYDRO-, (2R-(2.ALPHA.,3.ALPHA.,4.BETA.(2'R*,3'R*)))- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|