| 5655-61-8 |
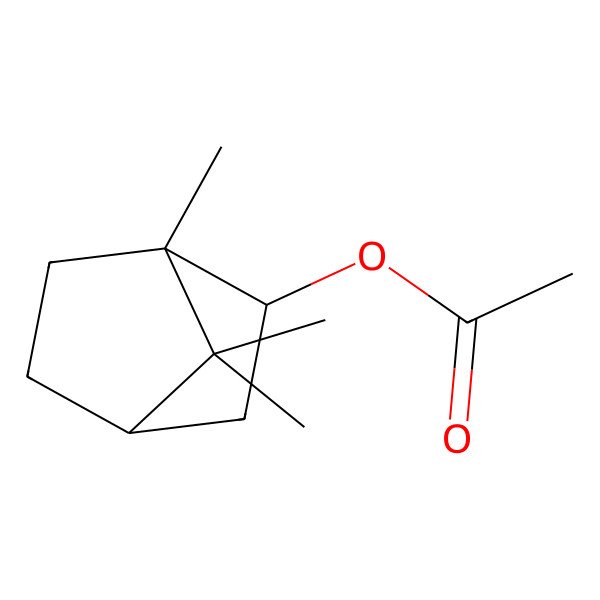
| BORNYL ACETATE |
| L-bornyl acetate |
| borneol acetate |
| 76-49-3 |
| (-)-Borneol acetate |
| Levo-bornyl acetate |
| Bornyl acetate [USAN] |
| Bornyl acetate, (-)- |
| UNII-24QAP1VCUX |
| 24QAP1VCUX |
| Borneol, acetate, (1S,2R,4S)-(-)- |
| (-)Bronyl acetate |
| laevo-bornyl acetate |
| (+/-)-Bornyl acetate |
| EINECS 227-101-4 |
| BORNYL ACETATE, L- |
| BORNYL ACETATE L-FORM |
| BORNEOL, L-, ACETATE |
| 1S-ENDO-BORNYL ACETATE |
| Bornyl ethanoate |
| DTXSID3052228 |
| FEMA NO. 4080 |
| BORNEOL ACETATE, (-)- |
| (1S,2R,4S)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-yl acetate |
| EC 227-101-4 |
| endo-bornyl acetate |
| Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S,2R,4S)- |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- |
| Bornyl acetic ether |
| 2-Camphanol acetate |
| 2-Camphanyl acetate |
| Bicyclo[2.2.1]heptan-2-ol,1,7,7-trimethyl-, 2-acetate, (1R,2S,4R)-rel- |
| 1,7,7-Trimethylbicyclo(2.2.1)heptan-2-ol acetate |
| FEMA No. 2159 |
| UNII-213431586X |
| L-Born-2-yl acetate |
| [(1S,2R,4S)-1,7,7-trimethyl-2-bicyclo[2.2.1]heptanyl] acetate |
| (-)-Bornyl acetate, 95% |
| 213431586X |
| endo-1,7,7-Trimethylbicyclo(2.2.1)hept-2-yl acetate |
| (1S)-1,7,7-TRIMETHYLBICYCLO(2.2.1)-2-HEPTANOL ACETATE |
| EINECS 200-964-4 |
| Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, 2-acetate, (1R,2S,4R)-rel- |
| Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, 2-acetate, (1S,2R,4S)- |
| Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, acetate, (1R,2S,4R)-rel- |
| BICYCLO(2.2.1)HEPTAN-2-OL, 1,7,7-TRIMETHYL-, ACETATE, (1S-ENDO)- |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S,2R,4S)- |
| NSC 407158 |
| AI3-00665 |
| Bicyclo(2.2.1)heptan-2-ol, 1,7,7-trimethyl-, acetate, endo- |
| DTXSID7041675 |
| Endo-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl acetate |
| L - born - 2 - yl acetate |
| 1,7,7-Trimethylbicyclo(2.2.1)heptan-2-yl acetate, endo- |
| Borneyl acetate |
| (1R,2S,4R)-rel-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol 2-acetate |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1R,2S,4R)-rel- |
| NSC-407158 |
| (1R,2S,4R)-REL-1,7,7-TRIMETHYLBICYCLO(2.2.1)HEPTAN-2-OL 2-ACETATE |
| (-)-Bornylacetat |
| (-)Bornyl acetate |
| L-.alpha.-bornyl acetate |
| 1,7,7-Trimethylbicyclo[2.2.1]hept-2-yl acetate # |
| BORNYL ACETATE [MI] |
| BORNYL ACETATE [FCC] |
| 1S-endo-Bornyl acetate; l-Bornyl acetate; levo-Bornyl acetate |
| BORNYL ACETATE [FHFI] |
| BORNYL ACETATE [INCI] |
| SCHEMBL132358 |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, endo- |
| Biphenyl-4-carbothioicacidamide |
| BORNYL ACETATE [MART.] |
| Bornyl acetate, >=98%, FG |
| (-)-[(1S)-bornyl]-acetate |
| BORNYL ACETATE [WHO-DD] |
| CHEMBL3186761 |
| DTXCID5030799 |
| L-BORNYL ACETATE [FHFI] |
| HY-N0756A |
| KGEKLUUHTZCSIP-HOSYDEDBSA-N |
| BORNYL ACETATE L-FORM [MI] |
| Tox21_303841 |
| [(1S,3R,4S)-4,7,7-trimethyl-3-bicyclo[2.2.1]heptanyl] acetate |
| s3893 |
| AKOS017343145 |
| CCG-266560 |
| DB14665 |
| (-)-Bornyl acetate, analytical standard |
| NCGC00357112-01 |
| AS-76314 |
| CAS-5655-61-8 |
| CS-0017052 |
| F16689 |
| (-)-BORNYL ACETATE (CONSTITUENT OF MYRRH) |
| Q27105264 |
| (-)-BORNYL ACETATE (CONSTITUENT OF MYRRH) [DSC] |
| [(1S,2R,4S)-1,7,7-Trimethylnorbornan-2-yl] acetate |
| BORNYL ACETATE (CONSTITUENT OF CHAMOMILE) [DSC] |
| F0001-1483 |
| (-)-Bornyl acetate, primary pharmaceutical reference standard |
| [(1S,4S,6R)-1,7,7-trimethyl-6-bicyclo[2.2.1]heptanyl] acetate |
|
There are more than 10 synonyms. If you wish to see them all click here.
|