| 520-26-3 |
| Cirantin |
| Hesperidoside |
| Hesperidine |
| Hesper bitabs |
| Hesperetin 7-rhamnoglucoside |
| Hesperetin 7-rutinoside |
| Hesperetin-rutinosid |
| Ciratin |
| Hesperidin, (2S)- |
| (S)-(-)-Hesperidin |
| USAF CF-3 |
| Hesperidin, (S)-(-)- |
| NSC 44184 |
| CCRIS 3940 |
| EINECS 208-288-1 |
| (2S)-Hesperidin |
| UNII-E750O06Y6O |
| BRN 0075140 |
| DIOSVEIN |
| Hesperetin-7-O-rhamnoglucoside |
| DTXSID9044328 |
| Hesperetin-7-rutinoside |
| CHEBI:28775 |
| E750O06Y6O |
| Hesperetin 7-O-rutinoside |
| Hesperitin-7-rhamnoglucoside |
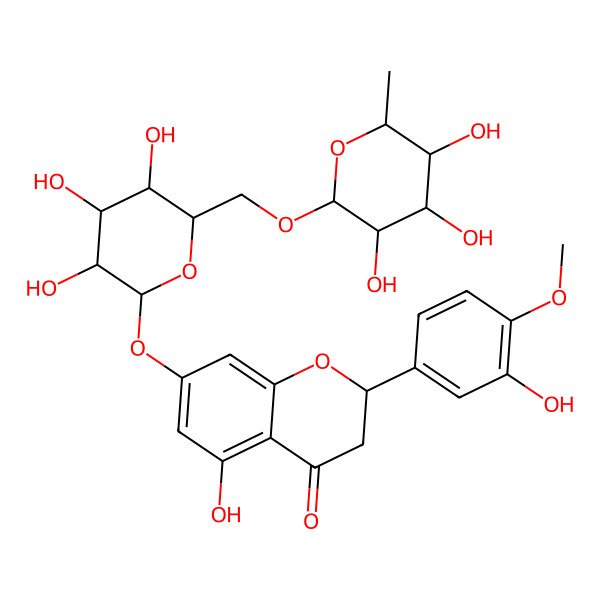
| (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one |
| 3',5'-Dihydroxy-4'-methoxy-7-rutinosyloxyflavan-4-on |
| MLS001304066 |
| DTXCID7024328 |
| EC 208-288-1 |
| 5-18-05-00218 (Beilstein Handbook Reference) |
| Hesperidin (JAN) |
| Hesperetin, 7-(6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside) |
| NSC-44184 |
| (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl 6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside |
| (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)chroman-4-one |
| 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydo-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (S)- |
| 5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((6-O-alpha-L-rhamnopyranosyl-beta-D-glucopyranosyl)oxy)-4-chromanon |
| 7-((6-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one, (S)- |
| 7-(6-O-Desoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyloxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-chromanon |
| SMR000718775 |
| HESPERIDIN [JAN] |
| MFCD00075663 |
| Glucopyranoside, hesperetin-7 6-O-(6-deoxy-alpha-L-mannopyranosyl)-, beta-D- |
| HESPERIDIN (MART.) |
| HESPERIDIN [MART.] |
| HESPERIDIN (USP-RS) |
| HESPERIDIN [USP-RS] |
| (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-3,4-dihydro-2H-1-benzopyran-4-one |
| (s)-7-[[6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one |
| 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (S)- |
| Aurantiamarin (Methyl Hesperidin) |
| 3',5'-DIHYDROXY-4'-METHOXY-7-RUTINOSYLOXYFLAVAN-4-ONE |
| hesperetin 7-(6-O-alpha-L-rhamnopyranosyl)-beta-D-glucopyranoside |
| NSC44184 |
| Hesperidin 2S |
| 2S, Hesperidin |
| (2S)-5-hydroxy-2-(3-hydroxy-4-methoxy-phenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl]oxymethyl]tetrahydropyran-2-yl]oxy-chroman-4-one |
| 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2S)- |
| Hesperitin-7-rutinoside |
| Hesperetin 7 Rutinoside |
| SR-01000799145 |
| Hesperetin 7 Rhamnoglucoside |
| Hesperidina |
| 7-Rhamnoglucoside, Hesperetin |
| Hesperidin,(S) |
| NCGC00016481-01 |
| (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl 6-O-(6-deoxy-a-L-mannopyranosyl)-ss-D-glucopyranoside |
| (2S)-7-[[6-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one (Hesperidin) |
| (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro-2H-pyran-2-yloxy)chroman-4-one |
| CAS-520-26-3 |
| Hesperetin Glycoside |
| C28H34O15 |
| HESPERIDEN Powder |
| Hesperidin, >=80% |
| HESPERIDIN [MI] |
| DIOSMIN [NDI] |
| Prestwick3_000400 |
| HESPERIDIN [INCI] |
| HESPERIDIN [VANDF] |
| D0I9HF |
| D0N6KM |
| HESPERIDIN [WHO-DD] |
| SCHEMBL94586 |
| BSPBio_000619 |
| cid_10621 |
| Hesperidin, analytical standard |
| BPBio1_000681 |
| CHEMBL449317 |
| NDI 590 [FDMS] |
| BCBcMAP01_000136 |
| BDBM61776 |
| NDI 590 |
| 2H-pyran-2-yloxy)chroman-4-one |
| QUQPHWDTPGMPEX-QJBIFVCTSA-N |
| HMS2096O21 |
| HMS2233I03 |
| HMS3713O21 |
| 4H-1-Benzopyran-4-one, 7-[[6-O-(6-deoxy-.alpha.-L-mannopyranosyl)-.beta.-D-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (S)- |
| Tox21_110448 |
| s2309 |
| AKOS015895450 |
| CCG-208580 |
| CS-5631 |
| CS-O-01636 |
| DB04703 |
| KS-5308 |
| C28-H34-O15 |
| SMP1_000149 |
| NCGC00179501-01 |
| HY-15337 |
| LS-74767 |
| AB00513829 |
| H0049 |
| C09755 |
| D01038 |
| (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)- |
| EN300-7419024 |
| Q385937 |
| Hesperidin, primary pharmaceutical reference standard |
| methyltetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro- |
| SR-01000799145-4 |
| SR-01000799145-5 |
| SR-01000799145-7 |
| 7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6- |
| BRD-K38903228-001-02-8 |
| BRD-K38903228-001-13-5 |
| Hesperidin, European Pharmacopoeia (EP) Reference Standard |
| Hesperidin, Pharmaceutical Secondary Standard; Certified Reference Material |
| Flavanone, 3',5,7-trihydroxy-4'-methoxy-, 7-(6-O-alpha-L-rhamnosyl-D-glucoside) |
| (2S)-2-(4-methoxy-3-oxidanyl-phenyl)-7-[(2S,3R,4S,5S,6R)-6-[[(2R,3R,4R,5R,6S)-6-methyl-3,4,5-tris(oxidanyl)oxan-2-yl]oxymethyl]-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5-oxidanyl-2,3-dihydrochromen-4-one |
| (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-2-oxanyl]oxymethyl]-2-oxanyl]oxy]-3,4-dihydro-2H-1-benzopyran-4-one |
| (2S)-7-((6-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-2,3-DIHYDRO-5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE |
| (2S)-7-((6-O-(6-DEOXY-alpha-L-MANNOPYRANOSYL)-beta-D-GLUCOPYRANOSYL)OXY)-2,3-DIHYDRO-5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE |
| (S)-7-[[6-O-(6-Deoxy-.alpha.-L-mannopyranosyl)-.beta.-D-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one |
| 4H-1-benzopirano-4-ona, 7-[[6-O-(6-deoxi-alfa-L-manopiranosil)-beta-D-glucopiranosil] oxi]-2,3-dihidro-5-hidroxi-2-(3-hidroxi-4-metoxifenil)-, (2S)- |
| 4H-1-BENZOPYRAN-4-ONE, 7-((6-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-2,3-DIHYDO-5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-, (S)- |
| 4H-1-Benzopyran-4-one, 7-[[6-O-(6-deoxy-.alpha.-L-mannopyranosyl)-.beta.-D-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2S)- |
| 5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-7-((6-O-.ALPHA.-L-RHAMNOPYRANOSYL-.BETA.-D-GLUCOPYRANOSYL)OXY)-4-CHROMANON |
|
There are more than 10 synonyms. If you wish to see them all click here.
|