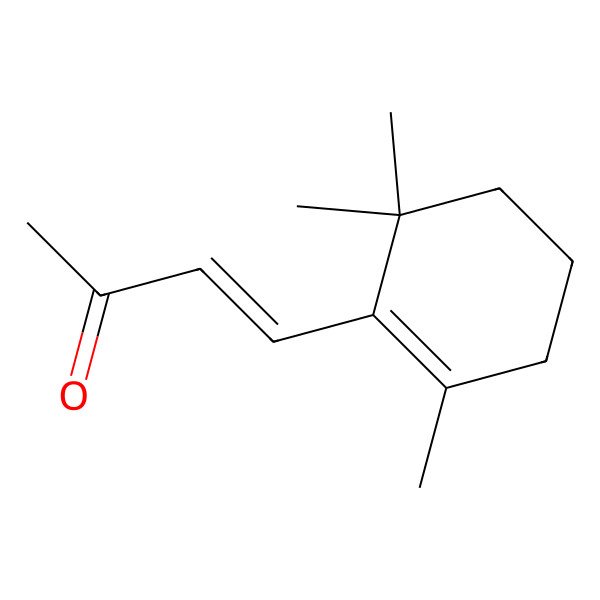
| 79-77-6 |
| 14901-07-6 |
| (E)-beta-Ionone |
| trans-beta-Ionone |
| 4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-one |
| 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one |
| (3E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-one |
| beta-Jonone |
| .beta.-Ionone |
| (E)-4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-one |
| beta-E-Ionone |
| beta-Cyclocitrylideneacetone |
| 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (3E)- |
| 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one |
| FEMA No. 2595 |
| CCRIS 6249 |
| NSC 402758 |
| b-ionone |
| beta-Ionon |
| ss-Ionone |
| (E)-4-(2,6,6-trimethylcyclohexen-1-yl)but-3-en-2-one |
| UNII-A7NRR1HLH6 |
| A7NRR1HLH6 |
| EINECS 238-969-9 |
| Ionone, .beta.- |
| 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- |
| CCRIS 4289 |
| beta-Ionone (trans) |
| trans-.beta.-Ionone |
| DTXSID4021769 |
| CHEBI:32325 |
| (E)-.beta.-Ionone |
| IONONE, BETA |
| EINECS 201-224-3 |
| EINECS 288-959-3 |
| NSC-46137 |
| 9-apo-beta-caroten-9-one |
| NSC-402758 |
| 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (E)- |
| BRN 1909544 |
| .beta.-Cyclocitrylideneacetone |
| AI3-25073 |
| DTXCID901769 |
| 4-(2,6,6-Trimethylcyclohex-1-ene-1-yl)-but-3-ene-2-one |
| (E)-4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one |
| HSDB 8269 |
| .beta.-Ionene |
| EC 201-224-3 |
| EC 238-969-9 |
| 2-07-00-00140 (Beilstein Handbook Reference) |
| NSC46137 |
| 4-(2,6,6-Trimethyl-1(or 2)-cyclohexen-1-yl)-3-buten-2-one |
| trans-4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one |
| 85949-43-5 |
| CAS-79-77-6 |
| WLN: L6UTJ A1U1V1 B1 F1 F1 |
| 4-(2,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one |
| ?-IONONE |
| 3-Buten-2-one,6,6-trimethyl-1-cyclohexen-1-yl)- |
| MFCD00001549 |
| 4-(2,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one |
| 4-(2,6-Trimethyl-1-cyclohexen-l-yl)-3-buten-2-one |
| DTXSID9025451 |
| [E]-4-[2,6,6-trimethyl-1-cyclohexen-1-yl]-3-buten-2-one |
| 4-[2,6,6-trimethyl-1(or 2)-cyclohexen-1-yl]-3-buten-2-one |
| beta ionone |
| beta -ionone |
| beta -E-ionone |
| Ionone, beta- |
| Nat. Beta Ionone |
| Trans-beta -ionone |
| (E)-beta -ionone |
| beta-Ionone, 96% |
| beta-Ionone, synthetic |
| BETA-IONONE [FCC] |
| (3E)-BETA-IONONE |
| 5,7-Megastigmadien-9-one |
| .beta.-Ionone isomer # 1 |
| .beta.-Ionone isomer # 2 |
| SCHEMBL23953 |
| .BETA.-IONONE [MI] |
| .BETA.-IONONE [FHFI] |
| US9144538, beta-Ionone |
| CHEMBL559945 |
| beta-Ionone, analytical standard |
| DTXCID3027952 |
| US9138393, ?-Ionone |
| FEMA 2595 |
| BETA-CYCLOCITRYLIDENACETONE |
| 3-BENZYLAMINO-PROPIONICACID |
| BDBM181139 |
| Tox21_201454 |
| Tox21_300709 |
| Tox21_302862 |
| BBL009828 |
| LS-871 |
| NSC402758 |
| STK801279 |
| beta-Ionone, natural, >=85%, FG |
| AKOS000121023 |
| CS-W015800 |
| HY-W015084 |
| beta-Ionone, purum, >=95.0% (GC) |
| NCGC00248145-01 |
| NCGC00248145-02 |
| NCGC00256534-01 |
| NCGC00257517-01 |
| NCGC00259005-01 |
| AM806748 |
| AS-68699 |
| VS-02204 |
| beta-Ionone, natural (US), >=85%, FG |
| CAS-14901-07-6 |
| EN300-18432 |
| D70747 |
| EN300-755077 |
| F81525 |
| beta-Ionone, predominantly trans, >=97%, FCC, FG |
| J-008542 |
| W-104258 |
| Q27114873 |
| 4-(2,6,6-Trimethyl-1-cyclohexen-l-yl)-3-buten-2-one |
| F0451-1336 |
| (E)-4-(2,6,6-trimethyl-1-cyclohexenyl)-but-3-en-2-one |
| (E)-4-(2,6,6-trimethylcyclohex-1-enyl)but-3-en-2-one |
| 4-(2,6,6-trimethyl-1-cyclohexene-1-yl)-3-buten-2-one |
| (3E)-4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one |
| (E)-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-on |
| 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-(E)-3-Buten-2-one |
| (3E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl) but-3-en-2-one |
| 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (E) |
| (E) - 4 - (2,6,6 - trimethyl - 1 - cyclohexen - 1 - yl) - 3 - buten - 2 - one |
| 4 - (2,6,6 - trimethylcyclohex - 1 - ene - 1 - yl) - but - 3 - ene - 2 - one |
| InChI=1/C13H20O/c1-10-6-5-9-13(3,4)12(10)8-7-11(2)14/h7-8H,5-6,9H2,1-4H3/b8-7 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|