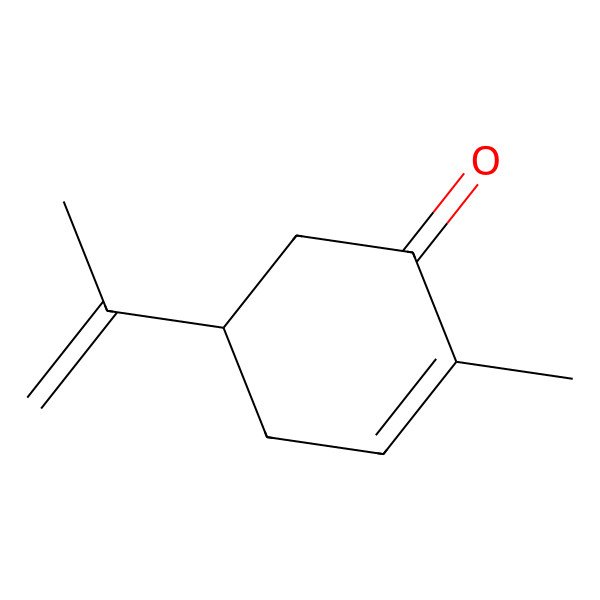
| 99-49-0 |
| 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-enone |
| Karvon |
| 1-Carvone |
| p-Mentha-6,8-dien-2-one |
| dl-Carvone |
| 2-Methyl-5-isopropenyl-2-cyclohexenone |
| Carvol |
| Carvone (natural) |
| Carvone [ISO] |
| (+/-)-carvone |
| D-Cavone |
| limonen-6-one |
| FEMA Number 2249 |
| 6,8(9)-p-Menthadien-2-one |
| 6,8-p-Menthadien-2-on |
| NCI-C55867 |
| 2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one |
| (+-)-Carvone |
| 2-Methyl-5-(1-methylethenyl)-2-cyclohexen-1-one |
| FEMA No. 2249 |
| 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)- |
| HSDB 707 |
| Carvon |
| UNII-75GK9XIA8I |
| NSC 6275 |
| EINECS 202-759-5 |
| 75GK9XIA8I |
| delta(sup 6,8)-(9)-terpadienone-2 |
| BRN 1364206 |
| CHEBI:38265 |
| AI3-08877 |
| p-mentha-1(6),8-dien-2-one |
| d-p-Mentha-1(6),8-dien-2-one |
| NSC-6275 |
| CARVONE, DL- |
| delta-1-Methyl-4-isopropenyl-6-cyclohexen-2-one |
| 6,8-p-Menthadien-2-one |
| 2-Methyl-5-(1-methylethenyl)-2-cyclohexene-1-one |
| 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one |
| C10H14O |
| CARVONE, (+-)- |
| CARVONE, (+/-)- |
| DTXSID8047426 |
| NSC6275 |
| (RS)-5-isopropenyl-2-methylcyclohex-2-en-1-one |
| 4-07-00-00316 (Beilstein Handbook Reference) |
| MFCD00062996 |
| Carvone 100 microg/mL in Acetonitrile |
| FEMA NO. 2249, (+/-)- |
| p-Mentha-6,8-dien-2-one, (R)-(-)- |
| 2-methyl-5-(1-methyl-1-ethenyl)-2-cyclohexen-1-one |
| 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-, (R)- |
| 5-isopropenyl-2-methylcyclohex-2-en-1-one |
| carvone, (R)-isomer |
| carvone, (+--)- |
| a carvone |
| MFCD00001578 |
| MFCD00062997 |
| .alpha.-Carvone |
| Spearmint terpenes |
| CARVONE DL-FORM |
| CARVONE [HSDB] |
| CARVONE [INCI] |
| CARVONE [MI] |
| 5-isopropenyl-2-methyl-cyclohex-2-en-1-one |
| 2-Methyl-5-(1-propen-2-yl)-2-cyclohexenone |
| NCIOpen2_001348 |
| SCHEMBL39408 |
| CARVONE DL-FORM [MI] |
| CHEMBL15676 |
| FEMA No. 3032 |
| CCRIS 7499 |
| EPA Pesticide Code 128800 |
| DTXCID6027426 |
| HSDB 1919 |
| AMY4152 |
| HMS1789N08 |
| d-p-mentha-1(6),8-dien-2-on |
| NSC93738 |
| Tox21_302547 |
| BBL010103 |
| NSC-93738 |
| STK801456 |
| AKOS000121377 |
| AKOS016843655 |
| LS-2355 |
| CAS-99-49-0 |
| NCGC00256915-01 |
| WLN: L6V BUTJ B1 EY1 & U1 |
| .delta.(sup 6,8)-(9)-Terpadienone-2 |
| AS-10471 |
| LS-89492 |
| NCI60_008753 |
| SY010704 |
| SY012922 |
| SY274718 |
| 2-Methyl-5-isopropenyl-2-cyclohexen-1-one |
| LS-145724 |
| CS-0033814 |
| FT-0600385 |
| FT-0605067 |
| FT-0658046 |
| EN300-16634 |
| O10834 |
| (-)-2-Methyl-5-isopropenyl-2-cyclohexen-1-one |
| 1-Methyl-4-isopropenyl-delta(6)-cyclohexen-2-one |
| 2-ciclohexen-1-ona, 2-metil-5-(1-metiletenil)- |
| A858458 |
| Q416800 |
| .delta.-1-Methyl-4-isopropenyl-6-cyclohexen-2-one |
| 5-Isopropenyl-2-methyl-2-cyclohexen-1-one, (R)- |
| W-100036 |
| Z56347241 |
| Flavor and Extract Manufacturers' Association Number 2249 |
| 22327-39-5 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|