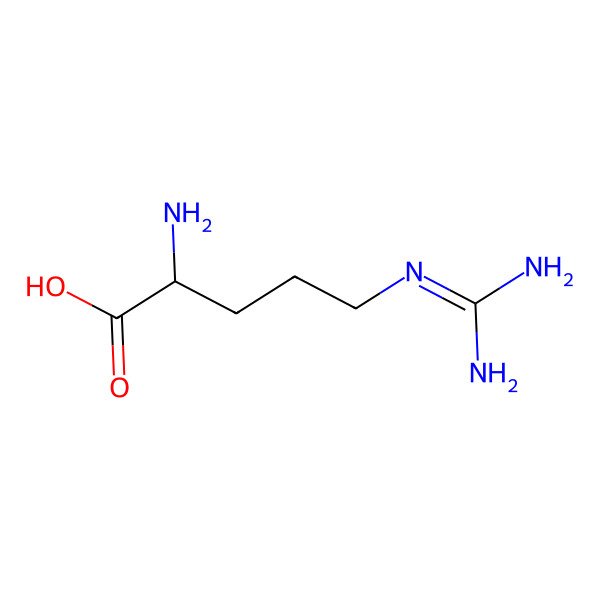
| arginine |
| 74-79-3 |
| L-(+)-Arginine |
| L(+)-Arginine |
| L-Arg |
| (S)-2-Amino-5-guanidinopentanoic acid |
| H-Arg-OH |
| (L)-Arginine |
| Arginina |
| ARGININE, L- |
| Arginine (VAN) |
| L-Arginin |
| Argininum [INN-Latin] |
| Arginina [INN-Spanish] |
| L-Ornithine, N5-(aminoiminomethyl)- |
| L-alpha-Amino-delta-guanidinovaleric acid |
| arg |
| 1-Amino-4-guanidovaleric acid |
| Argininum |
| CCRIS 3609 |
| (S)-(+)-arginine |
| NSC 206269 |
| Arginine [USAN:INN] |
| CHEBI:16467 |
| HSDB 1429 |
| AI3-24165 |
| (S)-2-Amino-5-((aminoiminomethyl)amino)pentanoic acid |
| (S)-2-Amino-5-guanidinovaleric acid |
| BRN 1725413 |
| Arginine [USP:INN] |
| L-Norvaline, 5-((aminoiminomethyl)amino)- |
| EINECS 200-811-1 |
| 2-amino-5-guanidinovaleric acid |
| CHEMBL1485 |
| UNII-94ZLA3W45F |
| (2S)-2-amino-5-guanidinopentanoic acid |
| (S)-2-Amino-5-[(aminoiminomethyl)amino]pentanoic acid |
| 94ZLA3W45F |
| DTXSID6041056 |
| (2S)-2-amino-5-carbamimidamidopentanoic acid |
| Pentanoic acid, 2-amino-5-((aminoiminomethyl)amino)-, (S)- |
| L-Arginine, labeled with tritium |
| (2S)-2-amino-5-(carbamimidamido)pentanoic acid |
| L-Arginine, monohydrochloride |
| NSC-206269 |
| C6H14N4O2 |
| Arginine (L-Arginine) |
| L-Norvaline, 5-[(aminoiminomethyl)amino]- |
| EC 200-811-1 |
| 4-04-00-02648 (Beilstein Handbook Reference) |
| (2S)-2-amino-5-(diaminomethylideneamino)pentanoic acid |
| DTXCID4021056 |
| Pentanoic acid, 2-amino-5-[(aminoiminomethyl)amino]-, (S)- |
| CAS-74-79-3 |
| 4455-52-1 |
| Arginine (Alternate RN) |
| NSC203450 |
| MFCD00002635 |
| 2-AMINO-5-GUANIDINO-PENTANOIC ACID |
| L-arginina |
| 3h-l-arginine |
| 1laf |
| L-a-Amino-d-guanidinovaleric acid |
| NCGC00015064-02 |
| (S)-Arginine |
| L(+) arginine |
| L-Aryginine,(S) |
| H-Arg |
| L-(+) arginine |
| L(+)-Arginine; |
| L-Arginine (9CI) |
| Arginine (USP/INN) |
| Tocris-0663 |
| (2S)-2-amino-5-guanidino-pentanoic acid |
| ARGININE [HSDB] |
| ARGININE [INCI] |
| L-Arginine (JP17) |
| ARGININE [INN] |
| ARGININE [II] |
| ARGININE [MI] |
| ARGININE [VANDF] |
| GND |
| Lopac-A-5006 |
| Arginine, L- (8CI) |
| ARGININE [MART.] |
| L-ARGININE [FCC] |
| L-ARGININE [JAN] |
| ARGININE [WHO-DD] |
| bmse000029 |
| bmse000899 |
| bmse000919 |
| D0F5DO |
| D0O3VV |
| Epitope ID:140084 |
| L-Arginine (H-Arg-OH) |
| L-ARGININE [FHFI] |
| SCHEMBL1791 |
| 2-amino-5-guanidinovalerate |
| Lopac0_000077 |
| Arginine hydrochloride(USAN) |
| GTPL721 |
| L-ARGININE [USP-RS] |
| L-a-Amino-d-guanidinovalerate |
| L-Amino-4-guanidovaleric acid |
| ARGININE [EP IMPURITY] |
| US9138393, L-Arginine |
| US9144538, L-Arginine |
| 1-Amino-4-guanidovalerlic acid |
| ARGININE [EP MONOGRAPH] |
| ARGININE [USP MONOGRAPH] |
| BDBM21959 |
| L-Arginine, 99%, FCC, FG |
| BDBM181132 |
| HMS3260O15 |
| N5-(aminoiminomethyl)-L-Ornithine |
| HY-N0455 |
| L-Arginine, Vetec(TM), 98.5% |
| Tox21_113046 |
| Tox21_500077 |
| AC-083 |
| L-2-Amino-5-guanidinopentanoic acid |
| L-alpha-Amino-delta-guanidinovalerate |
| L-Arginine, reagent grade, >=98% |
| s5634 |
| (C6-H14-N4-O2)x- |
| AKOS006239069 |
| AKOS015854096 |
| Tox21_113046_1 |
| AM81500 |
| CCG-204172 |
| DB00125 |
| LP00077 |
| SDCCGSBI-0050065.P002 |
| L-Arginine, 99%, natural, FCC, FG |
| (s)-2-amino-5-guanidino-pentanoic acid |
| 5-[(aminoiminomethyl)amino]-L-Norvaline |
| NCGC00015064-01 |
| NCGC00024715-01 |
| NCGC00024715-02 |
| NCGC00024715-03 |
| NCGC00024715-04 |
| NCGC00024715-05 |
| NCGC00024715-10 |
| NCGC00260762-01 |
| AS-14190 |
| LS-21576 |
| L-Arginine, BioUltra, >=99.5% (NT) |
| SBI-0207062.P001 |
| LS-170346 |
| LS-185750 |
| A0526 |
| EU-0100077 |
| L-Arginine, SAJ special grade, >=98.0% |
| EN300-73669 |
| A 5006 |
| C00062 |
| D02982 |
| L-Arginine, Vetec(TM) reagent grade, >=98% |
| LYSINE ACETATE IMPURITY F [EP IMPURITY] |
| M02981 |
| 2-azaniumyl-5-(diaminomethyleneammonio)pentanoate |
| AB00374192_03 |
| Norvaline, 5-[(aminoiminomethyl)amino]-, (L)- |
| A837397 |
| A929348 |
| Q173670 |
| SR-01000075479 |
| SR-01000597671 |
| (S)-2-amino-5-[(aminoiminomethyl)amino]-Pentanoate |
| (S)-2-Amino-5-[(aminoiminomethyl)amino]pentanoate |
| SR-01000075479-1 |
| SR-01000597671-1 |
| W-104410 |
| (S)-2-amino-5-[(aminoiminomethyl)amino]-Pentanoic acid |
| Arginine, European Pharmacopoeia (EP) Reference Standard |
| (2S)-2-amino-5-[(diaminomethylidene)amino]pentanoic acid |
| 7F15B0C7-356D-45D7-AC33-03AEE4394A0E |
| S-(+)-2-Amino-5-[(aminoiminomethyl)amino]pentanoic acid |
| (2S)-2-azanyl-5-[bis(azanyl)methylideneamino]pentanoic acid |
| L-Arginine, United States Pharmacopeia (USP) Reference Standard |
| L-Arginine, Pharmaceutical Secondary Standard; Certified Reference Material |
| 25212-18-4 |
| InChI=1/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/t4-/m0/s |
| L-Arginine, from non-animal source, meets EP, USP testing specifications, suitable for cell culture, 98.5-101.0% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|