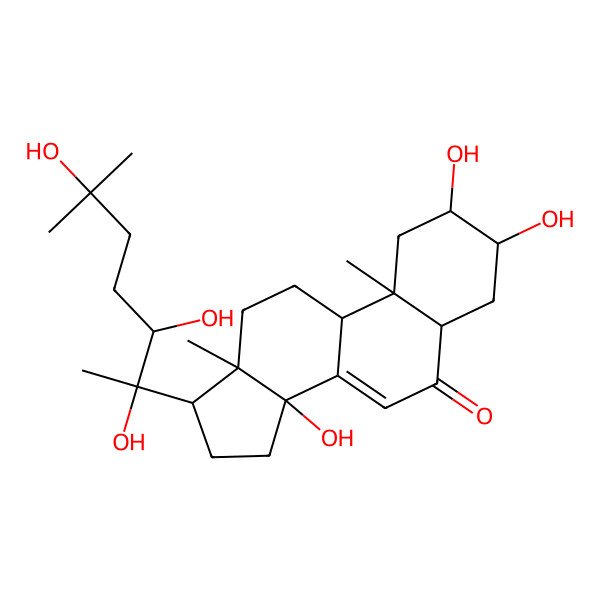
| Ecdysterone |
| 5289-74-7 |
| Crustecdysone |
| beta-Ecdysone |
| Commisterone |
| Isoinokosterone |
| Viticosterone |
| Crustecdyson |
| Ecdysteron |
| THE-7 |
| Ekdisten |
| 20-OH ecdysone |
| beta-ECDYSTERONE |
| Ecdysten |
| Hydroxyecdysone |
| beta-Ecdisone |
| b-Ecdysone |
| (+)-Ecdysterone |
| BIO101 |
| (2beta,3beta,5beta,22R)-2,3,14,20,22,25-hexahydroxycholest-7-en-6-one |
| UNII-779A7KPL0Y |
| 779A7KPL0Y |
| CHEMBL224128 |
| CHEBI:16587 |
| BIO-101 |
| C27H44O7 |
| 20-HYDROXY-.ALPHA.-ECDYSONE |
| NSC 629484 |
| NSC-629484 |
| 20E |
| BRN 1917578 |
| 20-E |
| AI3-44727 |
| Cholest-7-en-6-one, 2,3,14,20,22,25-hexahydroxy-, (2b,3b,5b,22R)- |
| Cholest-7-en-6-one, 2,3,14,20,22,25-hexahydroxy-, (2.beta.,3.beta.,5.beta.,22R)- |
| (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one |
| (2b,3b,5b,22R)-2,3,14,20,22,25-Hexahydroxycholest-7-en-6-one |
| 2-beta,3-beta,14,20,22,25-Hexahydroxy-5-beta-cholet-7-en-6-one |
| 2beta,3beta,14alpha,20,22R,25-hexahydroxy-5beta-cholest-7-en-6-one |
| MFCD00036740 |
| 2beta,3beta,14alpha,20R,22R,25-hexahydroxy-5beta-cholest-7-en-6-one |
| SMR000539832 |
| Edysterone |
| Ecdystene |
| Sarconeos |
| .beta-Ecdysterone |
| 20-Hydroxyecdyson |
| ?- Ecdysone |
| B-ecdysone,Commisterone |
| 20-Hydroxy-a-ecdysone |
| Bio 101 |
| SCHEMBL22086 |
| MLS001164644 |
| MLS002207226 |
| BIO 101 [WHO-DD] |
| DTXSID5040388 |
| GTPL11987 |
| 20-HYDROXYECDYSONE [MI] |
| 5-beta-Cholest-7-en-6-one, 2-beta,3-beta,14,20,22,25-hexahydroxy-, (22R)- |
| Cholest-7-en-6-one, 2,3,14,20,22,25-hexahydroxy-, (2-beta,3-beta,5-beta,22R)- |
| NKDFYOWSKOHCCO-YPVLXUMRSA-N |
| HMS2230L09 |
| HY-N6979 |
| 20-HYDROXYECDYSONE [WHO-DD] |
| BDBM50326777 |
| HB3718 |
| LMST01010209 |
| s2417 |
| AKOS004120029 |
| CCG-269554 |
| Cholest-7-en-6-one, 2,3,14,20,22,25-hexahydroxy-, (2beta,3beta,5beta,22R)- |
| AC-19603 |
| AC-34796 |
| CS-0007134 |
| 20-Hydroxyecdysone, >=93% (HPLC), powder |
| A829299 |
| Q423338 |
| Q-201054 |
| '2-beta,3-beta,14,20,22,25-Hexahydroxy-5-beta-cholet-7-en-6- one' |
| (2Beta,3beta,5beta)-2,3,14,20,22,25-hexahydroxycholest-7-en-6-one |
| '(2beta,3beta,5beta,22R)-2,3,14,20,22,25-hexahydroxycholest-7-en-6-one' |
| (22R)-2beta,3beta,14alpha,20,22,25-hexahydroxy-5beta-cholest-7-en-6-one |
| 5.beta.-Cholest-7-en-6-one, 2.beta.,3.beta.,14,20,22,25-hexahydroxy-, (22R)- |
| (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-((2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl)-1,2,3,4,5,9,10,11,12,13,14,15,16,17-tetradecahydro-6H-cyclopenta[a]phenanthren-6-one |
| (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(1R,2R)-1,2,5-trihydroxy-1,5-dimethyl-hexyl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one |
| 2,3,14-Trihydroxy-10,13-dimethyl-17-(1,2,5-trihydroxy-1,5-dimethyl-hexyl)-1,2,3,4,5,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-6-one |
|
There are more than 10 synonyms. If you wish to see them all click here.
|