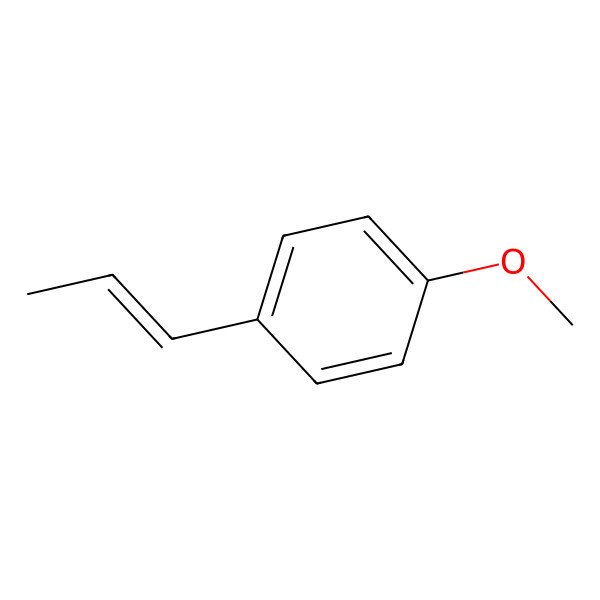
| trans-Anethole |
| 4180-23-8 |
| 104-46-1 |
| (E)-Anethole |
| p-Propenylanisole |
| (E)-1-Methoxy-4-(prop-1-en-1-yl)benzene |
| Anise camphor |
| Isoestragole |
| 4-Propenylanisole |
| Monasirup |
| cis-Anethol |
| Anethol |
| (E)-p-Propenylanisole |
| trans-p-Propenylanisole |
| trans-Anethol |
| p-Anethole |
| Anethole, trans |
| Anisole, p-propenyl- |
| (E)-Anethol |
| Aniskampfer |
| 1-Methoxy-4-(prop-1-en-1-yl)benzene |
| Anethole, trans- |
| trans-1-p-Anisylpropene |
| 1-methoxy-4-[(E)-prop-1-enyl]benzene |
| p-1-Propenylanisole |
| (E)-1-(4-Methoxyphenyl)propene |
| 1-Methoxy-4-propenylbenzene |
| 1-methoxy-4-[(1E)-prop-1-en-1-yl]benzene |
| 4-Methoxypropenylbenzene |
| trans-4-(1-Propenyl)anisole |
| trans-p-Anethole |
| FEMA No. 2086 |
| Benzene, 1-methoxy-4-(1-propenyl)- |
| (E)-1-Methoxy-4-(1-propenyl)benzene |
| trans-p-Methoxy-beta-methylstyrene |
| 1-Methoxy-4-(1-propenyl)benzene |
| 4-Methoxy-1-propenylbenzene |
| p-Propenylphenyl methyl ether |
| Propene, 1-(p-methoxyphenyl)- |
| t-anethole |
| trans-Anethole (natural) |
| Acintene O |
| 1-Propene, 1-(4-methoxyphenyl)- |
| Anethole (natural) |
| Anethole [USAN] |
| Benzene, 1-methoxy-4-(1-propenyl)-, (E)- |
| Propenylanisole, p-, (E)- |
| (E)-1-p-Methoxyphenylpropene |
| Anethol (synthetic) |
| Anisole, p-propenyl-, trans- |
| trans-1-Methoxy-4-(1-propenyl)benzene |
| Anisole, p-propenyl-, (E)- |
| CCRIS 2481 |
| Benzene, 1-methoxy-4-(1E)-1-propenyl- |
| 1-p-Methoxyphenylpropene, trans- |
| Caswell No. 051B |
| p-Propenylmethoxybenzene |
| FEMA Number 2086 |
| NSC 4018 |
| EINECS 224-052-0 |
| UNII-Q3JEK5DO4K |
| Methoxy-beta-methylstyrene, trans-p- |
| Q3JEK5DO4K |
| trans-1-(p-Methoxyphenyl)-1-propene |
| trans-1-(4-Methoxyphenyl)-1-propene |
| NSC 209529 |
| NSC-209529 |
| p-Methoxy-beta-methylstyrene |
| BRN 0636190 |
| UNII-A79C64YD3Q |
| 1-Methoxy-4-((1E)-1-propenyl)benzene |
| 1-Methoxy-4-(1-propenyl)benzene, (E)- |
| CCRIS 6211 |
| p-Methoxy-.beta.-methylstyrene |
| DTXSID9020087 |
| Trans-Anethole ((E)-Anethole) |
| CHEBI:35616 |
| HSDB 1427 |
| NSC4018 |
| Anethole (NF) |
| Anethole [NF] |
| Benzene, 1-methoxy-4-(propenyl)- |
| EINECS 203-205-5 |
| EINECS 256-753-2 |
| EPA Pesticide Chemical Code 015604 |
| BRN 0774229 |
| Benzene, 1-methoxy-4-(propen-1-yl)- |
| 1-Methoxy-4-(1E)-1-propen-1-ylbenzene |
| AI3-00380 |
| Benzene, 1-methoxy-4-(1-propen-1-yl)- |
| Methoxy-4-propenylbenzene |
| MFCD00009284 |
| (E)?-Anethole |
| EC 224-052-0 |
| DTXCID0086 |
| DTXCID2087 |
| 1-(methyloxy)-4-[(1E)-prop-1-en-1-yl]benzene |
| 2-06-00-00523 (Beilstein Handbook Reference) |
| E-anethole |
| trans-Anise camphor |
| 1-(4-Methoxyphenyl)-1(3)-propene |
| (E)-1-methoxy-4-(prop-1-enyl)benzene |
| p-(1-Propenyl)anisole |
| CAS-104-46-1 |
| 4-(1-propenyl)anisole |
| CAS-4180-23-8 |
| 1-(p-Methoxyphenyl)propene |
| SR-05000001866 |
| 4-06-00-03796 (Beilstein Handbook Reference) |
| CHEBI:2716 |
| DTXSID4020086 |
| trans-1-(p-Methoxyphenyl)propene |
| anethol- |
| 1-Methoxy-4-[1-propenyl]benzene # |
| Anisole, trans- |
| |trans|-Anethole |
| NCGC00091493-01 |
| 50770-19-9 |
| (E) - anethole |
| Anisole, 4-propenyl- |
| trans-Anethole, 99% |
| ANETHOLE [INCI] |
| ANETHOLE [FCC] |
| ANETHOLE [II] |
| ANETHOLE [MI] |
| 4-trans-propenyl-anisole |
| ANETHOLE [VANDF] |
| Spectrum5_000727 |
| ANETHOLE [USP-RS] |
| ANETHOLE [WHO-DD] |
| ANETHOLE, (E)- |
| ghl.PD_Mitscher_leg0.12 |
| UNII-21C2F5E8RE |
| SCHEMBL48599 |
| BSPBio_002818 |
| trans-p-Methoxypropenylbenzene |
| SPECTRUM1503705 |
| TRANS-ANETHOLE [FHFI] |
| WLN: 2U1R DO1 |
| Anethol, natural, 99%, FG |
| A79C64YD3Q |
| CHEMBL452630 |
| WLN: 2U1R DO1 -T |
| RUVINXPYWBROJD-ONEGZZNKSA-N |
| HMS1922I20 |
| HMS2089P20 |
| HMS2093I09 |
| HMS3885E16 |
| Pharmakon1600-01503705 |
| trans-Anethole, analytical standard |
| HY-B0900 |
| NSC-4018 |
| EINECS 288-194-5 |
| Tox21_111139 |
| Tox21_202001 |
| Tox21_202282 |
| Tox21_300132 |
| BBL027751 |
| CCG-38720 |
| NSC209529 |
| NSC758626 |
| s3779 |
| STK801277 |
| trans-p-Methoxy-.beta.-methylstyrene |
| trans-Anethole, >=99%, FCC, FG |
| AKOS000121299 |
| Tox21_111139_1 |
| DS-2756 |
| LS-2410 |
| NSC-758626 |
| methyl 4-(prop-1-en-1-yl)phenyl ether |
| NCGC00091493-02 |
| NCGC00091493-03 |
| NCGC00091493-04 |
| NCGC00091493-05 |
| NCGC00091493-06 |
| NCGC00091493-07 |
| NCGC00091493-09 |
| NCGC00254015-01 |
| NCGC00259550-01 |
| NCGC00259831-01 |
| 85681-56-7 |
| AC-34207 |
| Benzene, 1-methoxy-4(1E)-1-propenyl- |
| SBI-0052758.P002 |
| trans-Anethole, purum, >=98.0% (GC) |
| Benzene,1-Methoxy-4-(1-Propenyl)-,(E)- |
| P0494 |
| S3990 |
| Benceno, 1-metoxi-4-(1E)-1-propen-1-il- |
| EN300-18430 |
| A14711 |
| Benzene, 1-methoxy-4-(1E)-1-propen-1-yl- |
| D02377 |
| D70168 |
| AB00053256-02 |
| AB00053256_03 |
| EN300-1695718 |
| A873002 |
| Q255564 |
| (E)-ANETHOLE (CONSTITUENT OF MYRRH) [DSC] |
| Q-201853 |
| SR-05000001866-1 |
| SR-05000001866-2 |
| W-108812 |
| BRD-K49060658-001-01-5 |
| trans-Anethole, primary pharmaceutical reference standard |
| Z2315575049 |
| Anethole, United States Pharmacopeia (USP) Reference Standard |
| BENZENE,1-METHOXY,4-PROPENYL(TRANS) TRANS ANETHOL |
| InChI=1/C10H12O/c1-3-4-9-5-7-10(11-2)8-6-9/h3-8H,1-2H3/b4-3 |
| trans-Anethole, Pharmaceutical Secondary Standard; Certified Reference Material |
| 26795-32-4 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|