| 464-49-3 |
| (+)-Camphor |
| (R)-(+)-Camphor |
| (R)-Camphor |
| (1R)-(+)-Camphor |
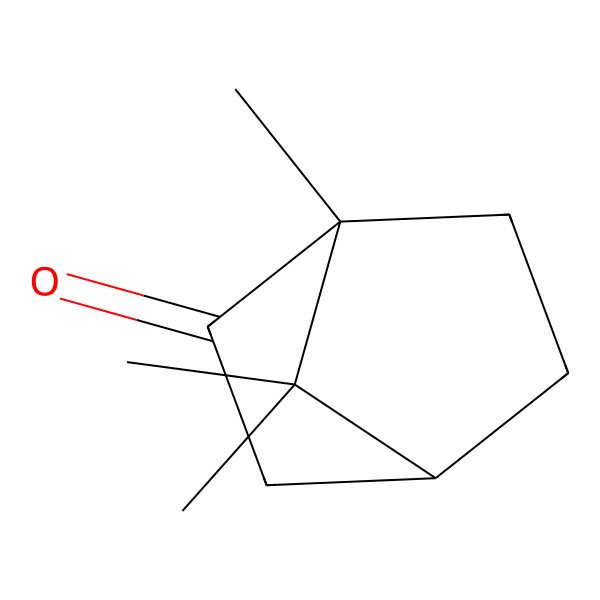
| (1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one |
| (+)-Bornan-2-one |
| Camphora |
| D(+)-Camphor |
| (1R,4R)-camphor |
| Camphor, Racemic |
| Camphor(D) |
| Dextrocamphor |
| Camphor (1R) |
| (+)-2-Bornanone |
| Natural camphor |
| Camphor D-form |
| camphor (natural) |
| Camphor, dl- |
| D-(+)-Camphor |
| Camphor, D- |
| FEMA No. 2230 |
| Camphor, (+)- |
| d-Camphor [JAN] |
| Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1R,4R)- |
| FEMA NO. 4513 |
| Camphor (synthetic) |
| NSC-755918 |
| 5TJD82A1ET |
| Alcanfor |
| SYNTHETIC CAMPHOR |
| UNII-N20HL7Q941 |
| Japanese camphor |
| DTXSID4024721 |
| CHEBI:15396 |
| Camphor USP |
| d-2-Camphanone |
| d-2-Bornanone |
| N20HL7Q941 |
| Bicyclo(2.2.1)heptane-2-one, 1,7,7-trimethyl- |
| (1R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one |
| (1R)-Camphor |
| (1R,4R)-4,7,7-trimethylbicyclo[2.2.1]heptan-3-one |
| NCGC00090681-04 |
| DTXCID804721 |
| (1R,4R)-1,7,7-Trimethylbicyclo(2.2.1)heptan-2-one |
| EINECS 207-355-2 |
| (-)-Alcanfor |
| UN2717 |
| (+-)-Camphor |
| CAMPHOR, RACEMIC (EP MONOGRAPH) |
| CAMPHOR, RACEMIC [EP MONOGRAPH] |
| CAS-464-49-3 |
| AI3-01698 |
| (+) - bornan - 2 - one |
| Camphorwood |
| Bicyclo(2.2.1)heptan-2-one, 1,7,7-trimethyl-, (1R,4R)- |
| Laurus camphora |
| Camphor laurel |
| EINECS 207-354-7 |
| NA2717 |
| NSC 26351 |
| Camphora camphora |
| D-(+)-Camphor;(1R)-(+)-Camphor |
| ?DL-Camphor |
| Cinnamonum camphora |
| 464-48-2 |
| 8008-51-3 |
| camphor-(+) |
| ( S)-camphor |
| Camphor, 96% |
| CAMPHORA [HPUS] |
| CAMPHOR [HSDB] |
| CAMPHOR [INCI] |
| CAMPHOR [MI] |
| Spectrum2_000383 |
| Spectrum3_000322 |
| CAMPHOR [USP-RS] |
| CAMPHOR [WHO-DD] |
| D-CAMPHOR [FHFI] |
| DL-CAMPHOR [JAN] |
| D-CAMPHOR [WHO-DD] |
| SCHEMBL16069 |
| BSPBio_001923 |
| CAMPHOR D-FORM [MI] |
| Bicyclo(2.2.1)heptan-2-one, 1,7,7-trimethyl-, (1theta)- |
| SPBio_000565 |
| (+)-CAMPHOR [FCC] |
| D-Camphor, >=97%, FG |
| CHEMBL504760 |
| BDBM36263 |
| KBio3_001143 |
| D-CAMPHOR [EP MONOGRAPH] |
| D-Camphor, natural, 96%, FG |
| Pharmakon1600-01500156 |
| (1R)-(+)-Camphor, 98% |
| (1R,4R)-(+)-CAMPHOR |
| HY-B1173 |
| Tox21_110995 |
| Tox21_202187 |
| Tox21_303052 |
| NSC755918 |
| (+/-)-Camphor, >=95.5% |
| AKOS016001972 |
| Tox21_110995_1 |
| CS-4779 |
| DB01744 |
| NSC 755918 |
| D-Camphor 1000 microg/mL in Methanol |
| D-Camphor, analytical reference material |
| NCGC00090681-01 |
| NCGC00090681-02 |
| NCGC00090681-06 |
| NCGC00090681-07 |
| NCGC00090730-03 |
| NCGC00257061-01 |
| NCGC00259736-01 |
| BS-42283 |
| (+/-)-Camphor, puriss., 96-101% |
| SBI-0206668.P002 |
| EN300-84949 |
| E75796 |
| (+/-)-Camphor, SAJ first grade, >=96.0% |
| AB00443849_03 |
| Camphor, synthetic [UN2717] [Flammable solid] |
| (+/-)-Camphor, purum, synthetic, >=95.0% (GC) |
| Q27089415 |
| Camphor (dl), primary pharmaceutical reference standard |
| Z1255434759 |
| Camphor, United States Pharmacopeia (USP) Reference Standard |
| (+)-Camphor, purum, natural, >=97.0% (sum of enantiomers, GC) |
| Camphor (racemic), European Pharmacopoeia (EP) Reference Standard |
| D-Camphor, Pharmaceutical Secondary Standard; Certified Reference Material |
| (+/-)-Camphor, meets analytical specification of Ph.??Eur., BP, racemic, >=95% (GC) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|