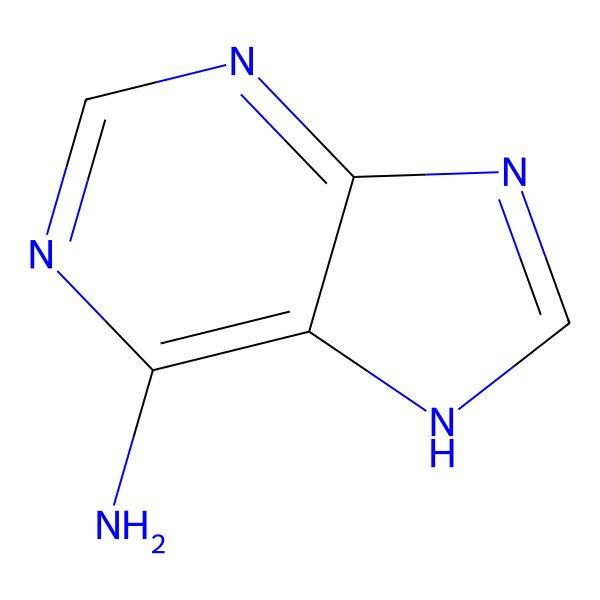
| 73-24-5 |
| 1H-Purin-6-amine |
| 6-Aminopurine |
| 7H-Purin-6-amine |
| 9H-Purin-6-amine |
| Vitamin B4 |
| Adenin |
| Adeninimine |
| Leuco-4 |
| 6-Amino-1H-purine |
| 6-Amino-3H-purine |
| 6-Amino-7H-purine |
| 6-Amino-9H-purine |
| 1,6-Dihydro-6-iminopurine |
| 3,6-Dihydro-6-iminopurine |
| Purine, 6-amino- |
| USAF CB-18 |
| 1H-Purine, 6-amino |
| Adenine [JAN] |
| ADE |
| 9H-Purine, 1,6-dihydro-6-imino- |
| Pedatisectine B |
| CCRIS 2556 |
| CHEBI:16708 |
| Vitamin B 4 |
| 3H-Purin-6(7H)-imine |
| 4, Vitamin B |
| B 4, Vitamin |
| 1H-Purine-6-amine |
| AI3-50679 |
| NSC 14666 |
| NSC-14666 |
| adenine-ring |
| EINECS 200-796-1 |
| MFCD00041790 |
| purine, 6 |
| 7H-Purin-6-amine (9CI) |
| ADENINE-2-D1 |
| UNII-JAC85A2161 |
| Leucon (TN) |
| Adenine (8CI) |
| 9H-Purine-6-amine |
| 9H-purin-6-ylamine |
| 134434-48-3 |
| 71660-29-2 |
| DTXSID6022557 |
| Adenine (JAN/USP) |
| Adenine [USP:JAN] |
| JAC85A2161 |
| 1H-Purine, 6-amino- |
| 66224-66-6 |
| 6H-Purin-6-imine, 3,9-dihydro-, (Z)- (9CI) |
| 1H-Purin-6-amine (9CI) |
| CHEMBL226345 |
| DTXCID502557 |
| 134434-49-4 |
| 134454-76-5 |
| NSC14666 |
| 6H-Purin-6-imine, 1,7-dihydro-, (Z)- (9CI) |
| 6H-Purin-6-imine, 1,9-dihydro-, (E)- (9CI) |
| 6H-Purin-6-imine, 3,7-dihydro-, (Z)- (9CI) |
| (Z)-3,9-Dihydro-6H-purin-6-imine |
| 134461-75-9 |
| NCGC00094856-01 |
| ADENINE (MART.) |
| ADENINE [MART.] |
| ADENINE (USP-RS) |
| ADENINE [USP-RS] |
| ADENINE (EP MONOGRAPH) |
| ADENINE (USP IMPURITY) |
| ADENINE [EP MONOGRAPH] |
| ADENINE [USP IMPURITY] |
| ADENINE (USP MONOGRAPH) |
| ADENINE [USP MONOGRAPH] |
| Adenine, United States Pharmacopeia (USP) Reference Standard |
| CAS-73-24-5 |
| ADENOSINE IMPURITY A (EP IMPURITY) |
| ADENOSINE IMPURITY A [EP IMPURITY] |
| SR-05000001754 |
| 6-Aminopurine (Adenine) |
| 1H-purin-6(9H)-imine |
| 1,6-Dihydro-6-Imnopurine |
| 3H-Purin-6-amine (9CI) |
| adenin- |
| 3h-adenine |
| 6-amino purine |
| 6-amino-Purine |
| purin-6-amine |
| 1jys |
| 1nli |
| 1wei |
| 2pqj |
| 3kpv |
| Vitamin-B4 |
| [3H]adenine |
| Adenine, 1 |
| Adenine,(S) |
| 9h-purin-6-amina |
| ALBB-005925 |
| 7H-purin-6-ylamine |
| 71660-30-5 |
| (S)-Norfluoxetine-d5 |
| 9H-Purin-6-yl-amin |
| Spectrum_001106 |
| 2p8n |
| starbld0001134 |
| 9H-Purin-6-yl-amine |
| ADENINE [VANDF] |
| SpecPlus_000535 |
| ADENINE [INCI] |
| Adenine, >=99% |
| 9H-Purin-6-amine # |
| ADENINE [MI] |
| Spectrum2_000583 |
| Spectrum3_000616 |
| Spectrum4_001891 |
| Spectrum5_000542 |
| ADENINE [WHO-DD] |
| 6-Aminopurine;Vitamin B4 |
| bmse000060 |
| bmse000861 |
| bmse000995 |
| D08IBS |
| Epitope ID:140097 |
| Adenine, cell culture grade |
| SCHEMBL8110 |
| Oprea1_057274 |
| US9138393, Adenine |
| US9144538, Adenine |
| BSPBio_002152 |
| KBioGR_002447 |
| KBioGR_002562 |
| KBioSS_001586 |
| KBioSS_002571 |
| MLS001066342 |
| DivK1c_006631 |
| SPECTRUM1500807 |
| SPBio_000426 |
| GTPL4788 |
| 9H-Purine,6-dihydro-6-imino- |
| 6,7-dihydro-3H-purin-6-imine |
| BDBM33218 |
| KBio1_001575 |
| KBio2_001586 |
| KBio2_002562 |
| KBio2_004154 |
| KBio2_005130 |
| KBio2_006722 |
| KBio2_007698 |
| KBio3_001652 |
| KBio3_003040 |
| 1,9-Dihydro-6H-purin-6-imine |
| Adenine 100 microg/mL in Water |
| cMAP_000085 |
| 7H-Purin-6-amine, min. 95% |
| BCPP000433 |
| BDBM181146 |
| HMS1921I14 |
| HMS2092K20 |
| HMS2269I04 |
| Pharmakon1600-01500807 |
| BCP02865 |
| HY-B0152 |
| VCA70030 |
| Tox21_111348 |
| Tox21_302108 |
| BBL007925 |
| BDBM50582699 |
| CCG-38506 |
| NSC757793 |
| s1981 |
| STK387542 |
| WLN: T56 BM DN FN HNJ IZ |
| AKOS000118903 |
| AKOS005171607 |
| Tox21_111348_1 |
| AC-2028 |
| AM83908 |
| BCP9000233 |
| CS-1984 |
| DB00173 |
| NSC-757793 |
| SDCCGMLS-0066584.P001 |
| NCGC00094856-02 |
| NCGC00094856-03 |
| NCGC00094856-05 |
| NCGC00255120-01 |
| AD2 |
| ANE |
| AS-11841 |
| BL008313 |
| NCI60_000998 |
| SMR000471871 |
| SBI-0052324.P002 |
| Adenine, Vetec(TM) reagent grade, >=99% |
| A0149 |
| FT-0620943 |
| FT-0656198 |
| EN300-21472 |
| Adenine, suitable for cell culture, BioReagent |
| C00147 |
| D00034 |
| P50008 |
| Q15277 |
| Z-1043 |
| AB00052833-18 |
| AB00052833-19 |
| AB00052833_20 |
| AB00052833_22 |
| AB00052833_23 |
| AB00052833_24 |
| A935233 |
| Q-200595 |
| SR-05000001754-1 |
| SR-05000001754-2 |
| W-106856 |
| Adenine, BioReagent, plant cell culture tested, >=99% |
| Adenine, European Pharmacopoeia (EP) Reference Standard |
| F0001-1848 |
| Z104498572 |
| 6379C0E0-C1BB-4087-96C5-1DE281B8EA4C |
| Adenine, Pharmaceutical Secondary Standard; Certified Reference Material |
| InChI=1/C5H5N5/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H3,6,7,8,9,10 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|