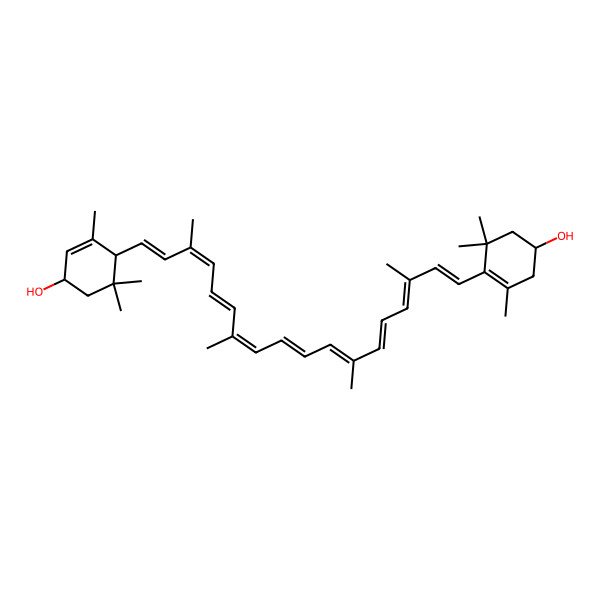
| XANTHOPHYLL |
| 127-40-2 |
| Bo-Xan |
| Vegetable lutein |
| Vegetable luteol |
| all-trans-Lutein |
| trans-Lutein |
| Xantofyl |
| Lutein ester |
| all-trans-(+)-Xanthophyll |
| FloraGLO |
| FloraGLO Lutein |
| Lutein A |
| Lutein, all-trans- |
| all-trans-Xanthophyll |
| Lutamax |
| Oro Glo 7 |
| Luteine |
| E 161b |
| Xanthophyll, all-trans-(+)- |
| Leutein |
| beta,epsilon-Carotene-3,3'-diol |
| Lutein from tagetes erecta |
| OS 24 |
| beta,epsilon-Carotene-3,3'-diol, (3R,3'R,6'R)- |
| UNII-X72A60C9MT |
| (3R,3'R,6'R)-Lutein |
| INS NO.161B(I) |
| X72A60C9MT |
| DTXSID8046749 |
| CHEBI:28838 |
| INS-161B(I) |
| E 161 |
| EINECS 204-840-0 |
| E-161B |
| E-161B(I) |
| NSC 59193 |
| NSC-59193 |
| DTXCID6026749 |
| (3R,3'R,6'R)-beta,epsilon-carotene-3,3'-diol |
| (3R,3'R)-dihydroxy-alpha-carotene |
| 180580-60-3 |
| .beta.,.epsilon.-Carotene-3,3'-diol |
| .beta.,.epsilon.-Carotene-3,3'-diol, (3R,3'R,6'R)- |
| LUTEIN (USP-RS) |
| LUTEIN [USP-RS] |
| LUTEIN (MART.) |
| LUTEIN [MART.] |
| (1R)-4-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1R,4R)-4-hydroxy-2,6,6-trimethylcyclohex-2-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethylcyclohex-3-en-1-ol |
| (1R,4R)-4-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-((R)-4-hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl)-3,5,5-trimethylcyclohex-2-enol |
| 9-cis-Lutein |
| C40H56O2 |
| NSC59193 |
| 13-cis-Lutein |
| gamma Lutein |
| Lutein, gamma |
| (3R,3'R,6R)-4,5-didehydro-5,6-dihydro-beta,beta-carotene-3,3'-diol |
| (1R,4R)-4-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-((4R)-4-HYDROXY-2,6,6-TRIMETHYL-1-CYCLOHEXEN-1-YL)-3,7,12,16-TETRAMETHYL-1,3,5,7,9,11,13,15,17-OCTADECANONAEN-1-YL)-3,5,5-TRIMETHYL-2-CYCLOHEXEN-1-OL |
| beta,?-carotene-3,3'-diol |
| Xanthophylls |
| Leutin |
| Beta, e- carotene- 3, 3'- diol |
| C40-H56-O2 |
| (3R,3'R,6S)-4,5-DIDEHYDRO-5,6-DIHYDRO-BETA,BETA-CAROTENE-3,3'-DIOL |
| NCGC00167965-01 |
| 15-cis-Lutein |
| ()-Lutein |
| 13'-cis-Lutein |
| Lutein (Xanthophyll) |
| (9'Z)-Lutein |
| Lutein - 5% |
| LEUTEIN [VANDF] |
| Xanthophyll (~80%) |
| LUTEIN [VANDF] |
| e-carotene-3,3'-diol |
| Lutein - 10% |
| Lutein - 20% |
| LUTEIN [DSC] |
| LUTEIN [FCC] |
| XANTHOPHYLL [MI] |
| Xanthophyll, from marigold |
| XANTOFYL [WHO-DD] |
| Lutein, analytical standard |
| SCHEMBL19342 |
| |A,|A-carotene-3,3'-diol |
| 3,3'-Dihydroxy-alpha-carotene |
| CHEMBL173929 |
| (invertedexclamationmarkA)-Lutein |
| BCBcMAP01_000190 |
| HMS3886I13 |
| 29414-89-9 |
| HY-N6947 |
| Tox21_112594 |
| ( inverted exclamation markA)-Lutein |
| BBL101804 |
| LMPR01070274 |
| MFCD08435941 |
| s5103 |
| STL555601 |
| AKOS008901394 |
| CCG-270087 |
| DB00137 |
| SMP1_000317 |
| AS-63011 |
| CAS-127-40-2 |
| XL176941 |
| XL176947 |
| XL176948 |
| CS-0015250 |
| C08601 |
| Q63409232 |
| 4',5'-Didehydro-6'-hydro-.beta.-carotene-3,3'-diol # |
| AB972DAC-E626-49F1-898D-598AF7729FD0 |
| (3R,3'R,6R)-4,5-DIDEHYDRO-5,6-DIHYDRO-beta,beta-CAROTIN-3,3'-DIOL |
| Lutein, Pharmaceutical Secondary Standard; Certified Reference Material |
| (3R,3'R,6R)-4,5-DIDEHYDRO-5,6-DIHYDRO-.BETA.,.BETA.-CAROTENE-3,3'-DIOL |
| (3R,3'R,6R)-4,5-DIDEHYDRO-5,6-DIHYDRO-.BETA.,.BETA.-CAROTIN-3,3'-DIOL |
| .BETA.,.BETA.-CAROTENE-3,3'-DIOL, 4,5-DIDEHYDRO-5,6-DIHYDRO-, (3R,3'R,6R)- |
| beta,beta-CAROTENE-3,3'-DIOL, 4,5-DIDEHYDRO-5,6-DIHYDRO-, (3R,3'R,6R)- |
| (1R)-4-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1R,4R)-4-hydroxy-2,6,6-trimethyl-cyclohex-2-en-1-yl]-3,7,12,16-tetramethyl-octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethyl-cyclohex-3-en-1-ol |
| 4-(18-(4-hydroxy-2,6,6-trimethyl-1-cyclohex-2-enyl)-3,7,12,16-tetramethyl-octadeca-1,3,5,7,9,11,13,15,17-nonaenyl)-3,5,5-trimethyl-cyclohex-3-en-1-ol |
|
There are more than 10 synonyms. If you wish to see them all click here.
|