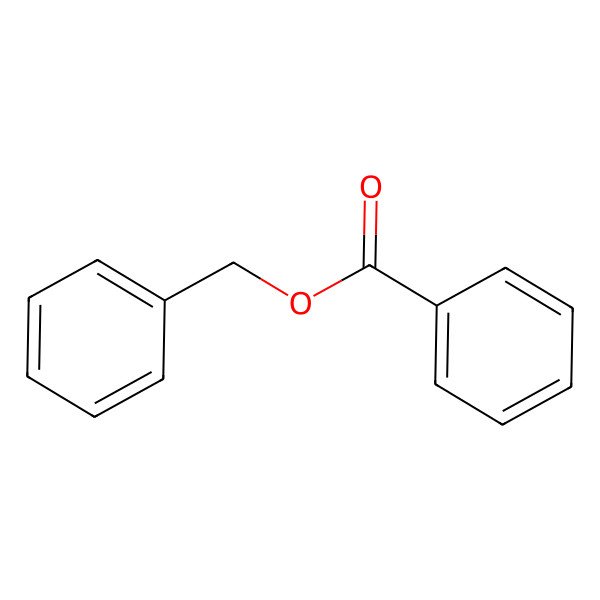
| 120-51-4 |
| Ascabiol |
| Novoscabin |
| Benylate |
| Benzoic acid, benzyl ester |
| Benzoic acid benzyl ester |
| Scabiozon |
| Scabitox |
| Scobenol |
| Ascabin |
| Benzoic acid, phenylmethyl ester |
| Phenylmethyl benzoate |
| Benzyl phenylformate |
| Benzylets |
| Peruscabin |
| Colebenz |
| Scabagen |
| Scabanca |
| Vanzoate |
| Scabide |
| Venzonate |
| Benzyl benzenecarboxylate |
| benzylbenzoate |
| Antiscabiosum |
| Benzylis benzoas |
| Benzyl alcohol benzoic ester |
| Peruscabina |
| Spasmodin |
| Benzylbenzenecarboxylate |
| Benzylum benzoicum |
| Benzoesaeurebenzylester |
| Caswell No. 082 |
| Benzylester kyseliny benzoove |
| BENZOIC ACID PHENYLMETHYLESTER |
| FEMA No. 2138 |
| FEMA Number 2138 |
| Benzyl benzoate (natural) |
| NSC 8081 |
| Acarobenzyl |
| Benzevan |
| HSDB 208 |
| NSC-8081 |
| EINECS 204-402-9 |
| Benzoate de benzyle |
| EPA Pesticide Chemical Code 009501 |
| UNII-N863NB338G |
| BRN 2049280 |
| DTXSID8029153 |
| CHEBI:41237 |
| AI3-00523 |
| N863NB338G |
| BENZYL-D5 BENZOATE |
| Benzylester kyseliny benzoove [Czech] |
| Benzyl benzoate [USP:JAN] |
| CHEMBL1239 |
| Benzyl benzoate [USAN:JAN] |
| BENZYLOXY PHENYL KETONE |
| Benzoic acid phenylmethyl ester |
| DTXCID809153 |
| EC 204-402-9 |
| 4-09-00-00307 (Beilstein Handbook Reference) |
| NCGC00094981-03 |
| BENZYL BENZOATE (II) |
| BENZYL BENZOATE [II] |
| BENZYL BENZOATE (MART.) |
| BENZYL BENZOATE [MART.] |
| BENZYL BENZOATE (USP-RS) |
| BENZYL BENZOATE [USP-RS] |
| Acarosan |
| Venzoate |
| Benzyl benzoate, analytical standard |
| BENZYL BENZOATE (EP IMPURITY) |
| BENZYL BENZOATE [EP IMPURITY] |
| BENZYL BENZOATE (EP MONOGRAPH) |
| BENZYL BENZOATE (USP IMPURITY) |
| BENZYL BENZOATE [EP MONOGRAPH] |
| BENZYL BENZOATE [USP IMPURITY] |
| BENZYL BENZOATE (USP MONOGRAPH) |
| BENZYL BENZOATE [USP MONOGRAPH] |
| 347840-01-1 |
| BZM |
| CAS-120-51-4 |
| SMR000471875 |
| benzylbenzoat |
| benzyl-benzoate |
| Benzyl benzoat |
| 1dzm |
| Benylate (TN) |
| benzoic acid benzyl |
| Benzoesaurebenzylester |
| MFCD00003075 |
| Spectrum_001240 |
| BENZOATE, BENZYL |
| Benzoic acid-benzyl ester |
| Spectrum2_000532 |
| Spectrum3_001757 |
| Spectrum4_000773 |
| Spectrum5_001128 |
| WLN: RVO1R |
| Benzoic acid, benzel ester |
| D0G1VX |
| Benzyl benzoate, >=99% |
| SCHEMBL3038 |
| BENZYL BENZOATE [MI] |
| BENZYL BENZOATE BP98 |
| BSPBio_003494 |
| KBioGR_001186 |
| KBioSS_001720 |
| MLS001066412 |
| MLS001336003 |
| MLS001336004 |
| BENZYL BENZOATE [FCC] |
| BENZYL BENZOATE [JAN] |
| DivK1c_000204 |
| SPECTRUM1503002 |
| SPBio_000543 |
| Benzyl benzoate (JP17/USP) |
| BENZYL BENZOATE [FHFI] |
| BENZYL BENZOATE [HSDB] |
| BENZYL BENZOATE [INCI] |
| BENZOIC ACID,BENZYL ESTER |
| HMS500K06 |
| KBio1_000204 |
| KBio2_001720 |
| KBio2_004288 |
| KBio2_006856 |
| KBio3_002714 |
| BENZYL BENZOATE [WHO-DD] |
| BENZYL BENZOATE [WHO-IP] |
| NSC8081 |
| NINDS_000204 |
| HMS1921P16 |
| HMS2092F20 |
| HMS2269D24 |
| Pharmakon1600-01503002 |
| HY-B0935 |
| Tox21_111372 |
| Tox21_201337 |
| Tox21_303418 |
| BDBM50134035 |
| CCG-39578 |
| NSC758204 |
| s4599 |
| STL183088 |
| BENZYL BENZOATE [ORANGE BOOK] |
| AKOS003495939 |
| BENZOIC ACID, PHENYLMETHYLESTER |
| Benzyl benzoate, >=99%, FCC, FG |
| Tox21_111372_1 |
| DB00676 |
| LS-2573 |
| NSC-758204 |
| BENZYLIS BENZOAS [WHO-IP LATIN] |
| IDI1_000204 |
| Benzyl benzoate, for synthesis, 99.0% |
| NCGC00094981-01 |
| NCGC00094981-02 |
| NCGC00094981-04 |
| NCGC00094981-05 |
| NCGC00094981-07 |
| NCGC00257502-01 |
| NCGC00258889-01 |
| AC-17033 |
| SBI-0051748.P002 |
| B0064 |
| FT-0622708 |
| Benzyl benzoate, natural, >=99%, FCC, FG |
| Benzyl benzoate, ReagentPlus(R), >=99.0% |
| Benzyl benzoate, SAJ first grade, >=98.0% |
| Benzyl benzoate, tested according to Ph.Eur. |
| A14577 |
| A19449 |
| Benzyl benzoate, SAJ special grade, >=99.0% |
| D01138 |
| AB00052298_07 |
| Benzyl benzoate, Vetec(TM) reagent grade, 98% |
| EN300-7323193 |
| Benzyl benzoate, meets USP testing specifications |
| Q413755 |
| SR-01000763773 |
| Benzoic acid-benzyl ester 5000 microg/mL in Hexane |
| Q-200696 |
| SR-01000763773-2 |
| BRD-K52072429-001-06-1 |
| Z19825582 |
| Benzoic acid-benzyl ester 100 microg/mL in Acetonitrile |
| Benzoic acid benzyl ester; Benzoic acid phenylmethyl ester |
| Benzyl benzoate, certified reference material, TraceCERT(R) |
| Benzyl benzoate, United States Pharmacopeia (USP) Reference Standard |
| Benzyl benzoate, Pharmaceutical Secondary Standard; Certified Reference Material |
| InChI=1/C14H12O2/c15-14(13-9-5-2-6-10-13)16-11-12-7-3-1-4-8-12/h1-10H,11H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|