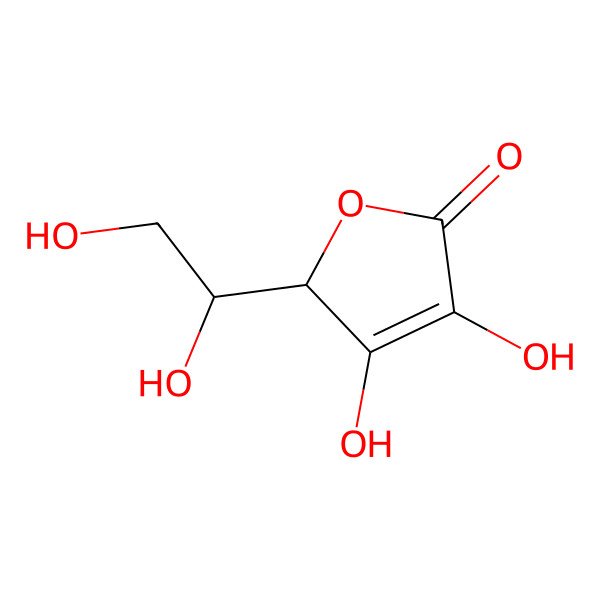
| l-ascorbic acid |
| vitamin C |
| 50-81-7 |
| ascorbate |
| L(+)-Ascorbic acid |
| L-ascorbate |
| Ascoltin |
| Ascorbicap |
| Cevitamic acid |
| Hybrin |
| Laroscorbine |
| Testascorbic |
| Allercorb |
| Ascorbajen |
| Ascorbutina |
| Ascorteal |
| Ascorvit |
| Cantaxin |
| Cebicure |
| Cegiolan |
| Celaskon |
| Cenetone |
| Cenolate |
| Cescorbat |
| Cetemican |
| Cevatine |
| Cevitamin |
| Citriscorb |
| Colascor |
| Concemin |
| Lemascorb |
| Proscorbin |
| Roscorbic |
| Viforcit |
| Viscorin |
| Vitacimin |
| Vitamisin |
| Vitascorbol |
| Adenex |
| Ascorb |
| Ascorin |
| Cantan |
| Cebion |
| Cebione |
| Ceglion |
| Cemagyl |
| Cemill |
| Cereon |
| Cergona |
| Cetamid |
| Cevalin |
| Cevimin |
| Cevital |
| Cevitan |
| Cevitex |
| Ciamin |
| Redoxon |
| Ribena |
| Vicelat |
| Vitace |
| Vitacee |
| Vitacin |
| Cebid |
| Cecon |
| Celin |
| Cevex |
| Cipca |
| Hicee |
| Xitix |
| Davitamon C |
| Arco-cee |
| Planavit C |
| Catavin C |
| Ce lent |
| Liqui-Cee |
| Vicomin C |
| Cee-Vite |
| Cevi-Bid |
| Natrascorb |
| Scorbacid |
| Scorbu-C |
| Secorbate |
| Duoscorb |
| C-Level |
| C-Vimin |
| Cetane-Caps TD |
| Cewin |
| Antiscorbic vitamin |
| C-Long |
| C-Quin |
| C-Span |
| Meri-C |
| Cee-Caps TD |
| L-Lyxoascorbic acid |
| L-Xyloascorbic acid |
| 3-Oxo-L-gulofuranolactone |
| Antiscorbutic vitamin |
| Ce-Mi-Lin |
| Natrascorb injectable |
| 3-Keto-L-gulofuranolactone |
| IDO-C |
| L-(+)-Ascorbic Acid |
| Cetane-Caps TC |
| Acidum ascorbinicum |
| CE-VI-Sol |
| Acidum ascorbicum |
| Kyselina askorbova |
| Dora-C-500 |
| Ferrous ascorbate |
| Ascor-B.I.D. |
| Ascorbicab |
| Acide ascorbique |
| (R)-5-((S)-1,2-dihydroxyethyl)-3,4-dihydroxyfuran-2(5H)-one |
| Cortalex |
| Ferancee |
| Stuartinic |
| Tolfrinic |
| Acido ascorbico |
| L-Threoascorbic acid |
| L-3-Ketothreohexuronic acid lactone |
| Caswell No. 061B |
| Antiscorbutic factor |
| FEMA No. 2109 |
| cevibid |
| Ascorbinsaeure |
| Chromagen |
| Magnorbin |
| Cetebe |
| Kyselina askorbova [Czech] |
| Ascor |
| L-threo-Ascorbic acid |
| NCI-C54808 |
| Ascorbicum acidum |
| L-threo-Hex-2-enonic acid, gamma-lactone |
| Acide ascorbique [INN-French] |
| Acido ascorbico [INN-Spanish] |
| Acidum ascorbicum [INN-Latin] |
| Semidehydroascorbate |
| 3-Oxo-L-gulofuranolactone (enol form) |
| Ascorbic acid, l- |
| Magnesium Ascorbicum |
| CCRIS 57 |
| (+)-Ascorbic acid |
| Monodehydroascorbic acid |
| Parentrovite |
| Chewcee |
| Citrovit |
| HSDB 818 |
| Juvamine |
| Sunkist |
| Ascorbic Acid, Monosodium Salt |
| roscorbi c |
| Vasc |
| vitamin-c |
| Acid Ascorbic |
| UNII-PQ6CK8PD0R |
| PQ6CK8PD0R |
| Rovimix C |
| Scorbu C |
| Oral Vitamin C |
| Hex-2-enonic acid gamma-lactone, L-threo- |
| ascor-b.i.d |
| Ascorbicap (TN) |
| EINECS 200-066-2 |
| NSC 33832 |
| Ascoltin (TN) |
| antiscorbic vita min |
| Vitamin c (as ascorbic acid) |
| Ronotec 100 |
| Ascorbyl radical |
| component of Cortalex |
| component of Ferancee |
| INS NO.300 |
| L-xylo-ascorbic acid |
| [14C]ascorbic acid |
| Ascorbic acid [BAN:INN:JAN] |
| Ascorbic acid [INN:BAN:JAN] |
| DTXSID5020106 |
| Rontex 100 |
| CHEBI:29073 |
| C6H8O6 |
| INS-300 |
| [14C]-ascorbic acid |
| ascorbic acid (vit C) |
| E-300 |
| L-threo-hex-2-enono-1,4-lactone |
| L-Ascorbic acid (GMP) |
| MFCD00064328 |
| NSC-33832 |
| Iron(II) ascorbate |
| NSC-218455 |
| L-3-ketothreohexuronic acid |
| component of E and C-Level |
| component of Endoglobin Forte |
| E300 |
| DTXCID90106 |
| Sodium Ascorbate (Ascorbic Acid) |
| (5R)-5-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-2,5-dihydrofuran-2-one |
| L-Ascorbic acid, free radical form |
| Ascorbic acid [USP:INN:BAN:JAN] |
| NSC 218455 |
| 6730-29-6 |
| NCGC00164357-01 |
| Xyloascorbic acid, L- |
| Ester-C |
| (2R)-2-[(1S)-1,2-dihydroxyethyl]-4,5-dihydroxyfuran-3-one |
| (2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one |
| (5R)-5-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one |
| hex-1-enofuranos-3-ulose |
| ASCORBIC ACID (II) |
| ASCORBIC ACID [II] |
| L-Ascorbic acid 1000 microg/mL in Acetonitrile |
| 2,3-DEHYDRO-L-THREO-HEXONO-1,4-LACTONE |
| Ascorbinsaure |
| Kangbingfeng |
| 2-(1,2-Dihydroxyethyl)-4,5-dihydroxyfuran-3-one |
| Ceklin |
| CALCIUM ASCORBATE |
| ASCORBIC ACID (MART.) |
| ASCORBIC ACID [MART.] |
| Acido ascorbico (INN-Spanish) |
| Acidum ascorbicum (INN-Latin) |
| Cell C |
| (2R)-2-[(1S)-1,2-Dihydroxyethyl]-4,5-dihydroxy-furan-3-one |
| NSC33832 |
| Viscorin 100M |
| ASCORBIC ACID (EP MONOGRAPH) |
| ASCORBIC ACID [EP MONOGRAPH] |
| ASCORBIC ACID (USP MONOGRAPH) |
| ASCORBIC ACID [USP MONOGRAPH] |
| Suncoat VC 40 |
| Acid, Ascorbic |
| (5R)-5-((1S)-1,2-DIHYDROXYETHYL)-3,4-DIHYDROXYFURAN-2(5H)-ONE |
| L Ascorbic Acid |
| Acid, L-Ascorbic |
| 53262-66-1 |
| L-Ascorbic acid, meets USP testing specifications |
| DTXSID7048112 |
| Ascorbinezuur |
| Cevitamate |
| Asorbicap |
| L-lyxoascorbate |
| L-xyloascorbate |
| .Ascorbinsaure |
| C Vitamin |
| 3eka |
| cee-caps td |
| Ester C |
| l-threo-hex-1-eofuranos-3-ulose |
| (+)-ascorbate |
| L(+)-ascorbate |
| L-(+)-ascorbate |
| Vitamin C,(S) |
| E 300 |
| NEO-VALDRIN |
| Ascorbic Acid DC97SF |
| VIT C |
| Vitamin xyloascorbic acid |
| Prestwick3_000325 |
| L-Ascorbic acid, 99% |
| ASCOR (TN) |
| SCHEMBL785 |
| bmse000182 |
| D06NNE |
| D07AHW |
| D0U4HT |
| VITAMIN C [VANDF] |
| Vitamin C (Ascorbic acid) |
| ASCORBIC ACID [MI] |
| SCHEMBL4430 |
| ASCORBIC ACID [FCC] |
| ASCORBIC ACID [INN] |
| ASCORBIC ACID [JAN] |
| L-Ascorbic acid, FCC, FG |
| L-Ascorbic Acid, Free Acid |
| ASCORBIC ACID [FHFI] |
| ASCORBIC ACID [HSDB] |
| ASCORBIC ACID [INCI] |
| BSPBio_000329 |
| (r)-5-(1,2-dihydroxy-ethyl)-3,4-dihydroxy-5h-furan-2-one |
| MLS002153776 |
| ASCORBIC ACID [VANDF] |
| CHEMBL40274 |
| L-Ascorbic acid, cell culture |
| L-(+)-ACSCORBIC ACID |
| BPBio1_000363 |
| GTPL4532 |
| GTPL4781 |
| L-Ascorbic acid, reagent grade |
| ASCORBIC ACID [USP-RS] |
| ASCORBIC ACID [WHO-DD] |
| ASCORBIC ACID [WHO-IP] |
| L-Ascorbic acid, >=99.0% |
| CHEBI:22652 |
| HY-B0166G |
| DTXCID50820452 |
| DTXSID50986567 |
| Ascorbic acid (JP15/USP/INN) |
| Ascorbic acid (JP17/USP/INN) |
| CIWBSHSKHKDKBQ-JLAZNSOCSA-N |
| HMS2096A11 |
| HMS2231N16 |
| HMS3713A11 |
| L-Ascorbic acid ACS reagent grade |
| ASCORBIC ACID [ORANGE BOOK] |
| BCP27915 |
| HY-B0166 |
| L-Threoascorbic acid,Antiscorbutic factor,Vitamin C;(R)-5-((S)-1,2-Dihydroxyethyl)-3,4-dihydroxyfuran-2(5H)-one |
| Tox21_110315 |
| Tox21_112104 |
| Tox21_202127 |
| Tox21_302958 |
| gamma-lactone L-threo-Hex-2-enonate |
| HB1238 |
| L-Ascorbic acid, analytical standard |
| L-Ascorbic acid, AR, >=99.5% |
| LS-145 |
| s3114 |
| AKOS016843589 |
| Tox21_112104_1 |
| CCG-207946 |
| CS-O-00843 |
| DB00126 |
| L-Ascorbic acid, mixt. with vitamin B |
| ACIDUM ASCORBICUM [WHO-IP LATIN] |
| gamma-lactone L-threo-Hex-2-enonic acid |
| L-Ascorbic acid, ACS reagent, >=99% |
| NCGC00091517-01 |
| NCGC00091517-02 |
| NCGC00091517-03 |
| NCGC00091517-06 |
| NCGC00188972-01 |
| NCGC00256504-01 |
| NCGC00259676-01 |
| BP-12831 |
| SMR001233160 |
| L-Ascorbic acid, plant cell culture tested |
| L-Ascorbic acid, reagent grade, crystalline |
| A0537 |
| AB00376923 |
| Ascorbic Acid (L-Ascorbic Acid; Vitamin C) |
| CS-0626121 |
| SW198791-2 |
| L-3-KETO-THREO-HEXURONIC ACID LACTONE |
| L-Ascorbic acid, BioUltra, >=99.5% (RT) |
| L-Ascorbic acid, tested according to Ph.Eur. |
| C00072 |
| D00018 |
| E80759 |
| EN300-708766 |
| L-Ascorbic acid, p.a., ACS reagent, 99.0% |
| AB00376923_04 |
| AB00376923_05 |
| L-Ascorbic acid, JIS special grade, >=99.0% |
| L-Ascorbic acid, Vetec(TM) reagent grade, 99% |
| L-Ascorbic acid, BioXtra, >=99.0%, crystalline |
| Q199678 |
| 2-(1,2-dihydroxyethyl)-4,5-dihydroxy-furan-3-one |
| L-Ascorbic acid, puriss. p.a., >=99.0% (RT) |
| Q27101942 |
| 47A605F0-4187-47A8-B0CE-F9E7DA1B0076 |
| L-Ascorbic acid, p.a., ACS reagent, reag. ISO, 99.7% |
| L-Threo-2,3,4,5,6-pentahydroxy-1-hexenoicacid-4-lactone |
| Ascorbic acid, British Pharmacopoeia (BP) Reference Standard |
| Ascorbic acid, European Pharmacopoeia (EP) Reference Standard |
| L-Ascorbic acid, certified reference material, TraceCERT(R) |
| L-Ascorbic acid, powder, cell culture tested, gamma-irradiated |
| 3,4-Dihydroxy-5beta-[(S)-1,2-dihydroxyethyl]furan-2(5H)-one |
| Ascorbic acid, United States Pharmacopeia (USP) Reference Standard |
| (2R)-2-[(1S)-1,2-dihydroxyethyl]-4,5-dihydroxy-2,3-dihydrofuran-3-one |
| 4-((E)-2-[(2-HYDROXYETHYL)SULFANYL]DIAZENYL)BENZENECARBOXYLICACID |
| L-Ascorbic acid, anhydrous, free-flowing, Redi-Dri(TM), ACS reagent, >=99% |
| L-Ascorbic acid, suitable for cell culture, suitable for plant cell culture, >=98% |
| 14536-17-5 |
| L-Ascorbic acid, puriss. p.a., ACS reagent, reag. ISO, reag. Ph. Eur., 99.7-100.5% (oxidimetric) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|