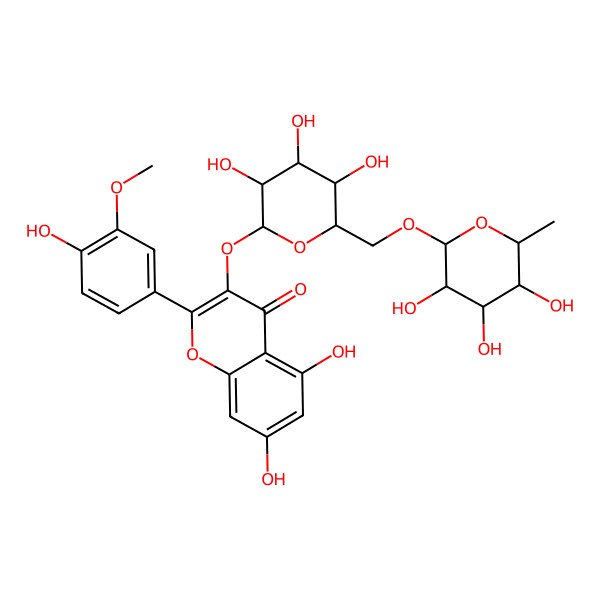
| Narcissin |
| 604-80-8 |
| Isorhamnetin 3-rutinoside |
| Narcissin Flavonol |
| Isorhamnetin-3-O-rutinoside |
| Isorhamnetin 3-O-rutinoside |
| Isorhamnetin 3-rhamnoglucoside |
| Isprhamnetin-3-rutinoside |
| UNII-N4AX11L1TF |
| N4AX11L1TF |
| C28H32O16 |
| 5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one |
| 3-O-RUTINOSYLISORHAMNETIN |
| DTXSID00209157 |
| 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one |
| 3'-O-METHYLQUERCETIN 3-RUTINOSIDE |
| 4H-1-Benzopyran-4-one, 3-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)- |
| 4H-Benzopyran-4-one, 3-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)- |
| Isorhamnetin-3-rutinoside |
| ISORHAMNETIN-3-O-RUTINOSIDE (USP-RS) |
| ISORHAMNETIN-3-O-RUTINOSIDE [USP-RS] |
| Isorhamnetin3-rutinoside |
| Narcissin (6CI,7CI,8CI) |
| CHEMBL258394 |
| SCHEMBL7167500 |
| DTXCID20131648 |
| CHEBI:145096 |
| HY-N0649 |
| BDBM50379284 |
| MFCD00017734 |
| s9423 |
| AKOS030632878 |
| CCG-270266 |
| ISORHAMNETIN 3-beta-O-RUTINOSIDE |
| ISORHAMNETIN 3-O-beta-RUTINOSIDE |
| AC-34730 |
| AS-75139 |
| LS-39514 |
| ISORHAMNETIN 3-.BETA.-O-RUTINOSIDE |
| ISORHAMNETIN 3-O-.BETA.-RUTINOSIDE |
| CS-0009675 |
| ISORHAMENTIN-3-O-.BETA.-D-RUTINOSIDE |
| C16060 |
| Q23418564 |
| ISORHAMNETIN-3-O-RUTINOSIDE (CONSTITUENT OF GINKGO) |
| ISORHAMNETIN-3-O-RUTINOSIDE (CONSTITUENT OF GINKGO) [DSC] |
| ISORHAMNETIN 3-O-.BETA.-D-(6-O-.ALPHA.-L-RHAMNOSYL)GLUCOSIDE |
| 4H-1-BENZOPYRAN-4-ONE, 3-996-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-5,7-DIHYDROXY-2-(4-HYDROXY-3-METHOXYPHENYL)- |
| 4H-1-BENZOPYRAN-4-ONE, 3-996-O-(6-DEOXY-alpha-L-MANNOPYRANOSYL)-beta-D-GLUCOPYRANOSYL)OXY)-5,7-DIHYDROXY-2-(4-HYDROXY-3-METHOXYPHENYL)- |
| 4H-1-Benzopyran-4-one,3-[[6-O-(6-deoxy-a-L-mannopyranosyl)-b-D-glucopyranosyl]oxy]-5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)- |
| 5,7-dihydroxy-2-(4-hydroxy-3-methoxy-phenyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl]oxymethyl]tetrahydropyran-2-yl]oxy-chromen-4-one |
| 5,7-Dihydroxy-2-(4-hydroxy-3-methoxy-phenyl)-3-[3,4,5-trihydroxy-6-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxymethyl)-tetrahydro-pyran-2-yloxy]-1-benzopyran-4-one |
| 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one |
| 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-4H-chromen-4-one |
|
There are more than 10 synonyms. If you wish to see them all click here.
|