| 57-48-7 |
| D(-)-Fructose |
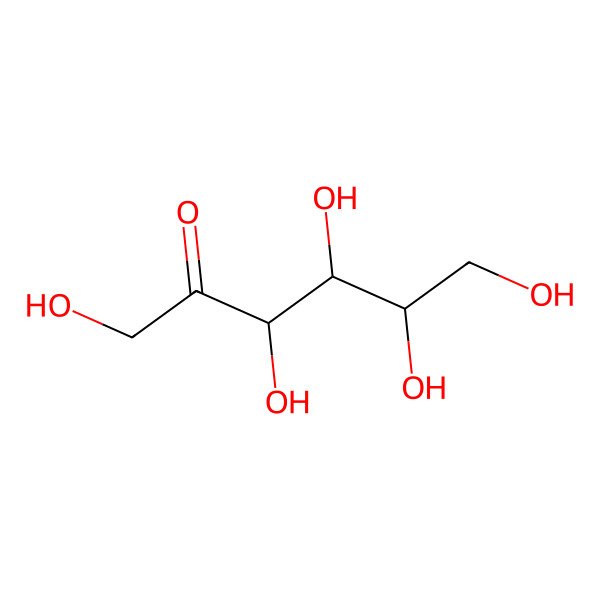
| (3S,4R,5R)-1,3,4,5,6-pentahydroxyhexan-2-one |
| Nevulose |
| D-Levulose |
| DL-Fructose |
| 30237-26-4 |
| Furucton |
| Methose |
| D-(-)-Levulose |
| arabino-Hexulose |
| Sugar, fruit |
| Fructose, D- |
| arabino-2-Hexulose |
| Fructose [JAN] |
| Krystar 300 |
| Hi-Fructo 970 |
| keto-D-fructose |
| Fructose, pure |
| Advantose FS 95 |
| CCRIS 3335 |
| (+-)-Fructose |
| Fructose [USP:JAN] |
| EINECS 200-333-3 |
| UNII-6YSS42VSEV |
| 6YSS42VSEV |
| AI3-23514 |
| DTXSID5023081 |
| UNII-02T79V874P |
| CHEBI:48095 |
| 02T79V874P |
| rel-(3S,4R,5R)-1,3,4,5,6-Pentahydroxyhexan-2-one |
| D-(-)-Fructose, >=99% |
| CAS-57-48-7 |
| D-(-)fructose |
| MFCD00148910 |
| alpha-Acrose |
| D-fructose-ring |
| D-Fructosa |
| NCGC00160604-01 |
| Fructose (VAN) |
| Fructose,(S) |
| FUD |
| Fructon (TN) |
| D(-)-ructose |
| D-Fructose,(S) |
| pentahydroxyhexan-2-one |
| FRUCTOSE [INCI] |
| .ALPHA.-ACROSE |
| FRUCTOSE [FCC] |
| FRUCTOSE [MI] |
| FRUCTOSE, DL- |
| D-(-)-Fructose, LR |
| Fructose (JP17/USP) |
| DL-FRUCTOSE [MI] |
| Topiramate impurity E CRS |
| D02OIY |
| D06HZY |
| FRUCTOSE [WHO-DD] |
| SCHEMBL3965 |
| D-(-)-Fructose, BioXtra |
| D-(-)-Fructose, puriss. |
| D-fructose (open structure) |
| (+/-)-FRUCTOSE |
| GTPL4654 |
| CHEMBL1232863 |
| FRUCTOSE, (+/-)- |
| BJHIKXHVCXFQLS-UYFOZJQFSA-N |
| 2C6H12O6 |
| HY-N7092 |
| Tox21_113557 |
| Tox21_200762 |
| s5176 |
| 1,3,4,5,6-Pentahydroxyhex-2-one |
| AKOS015901521 |
| NSC 760385 |
| GLUCOSE IMPURITY D [EP IMPURITY] |
| NCGC00258316-01 |
| LS-69766 |
| LACTULOSE IMPURITY D [EP IMPURITY] |
| CS-0008532 |
| D-(-)-Fructose, for microbiology, >=99.0% |
| D-(-)-Fructose, tested according to Ph.Eur. |
| D00114 |
| EN300-218371 |
| A870797 |
| D-(-)-Fructose, BioUltra, >=99.0% (HPLC) |
| D-(-)-Fructose, meets USP testing specifications |
| D-(-)-Fructose, SAJ special grade, >=98.0% |
| Q122043 |
| TOPIRAMATE IMPURITY, FRUCTOSE- [USP IMPURITY] |
| (3S,4R,5R)-1,3,4,5,6- |
| DFA8C62B-E34B-4603-A548-F6A8D25645DD |
| Fructose, European Pharmacopoeia (EP) Reference Standard |
| Z1255372738 |
| (3S,4R,5R)-1,3,4,5,6-pentakis(oxidanyl)hexan-2-one |
| Fructose, United States Pharmacopeia (USP) Reference Standard |
| D-(-)-Fructose, meets analytical specification of Ph.??Eur., BP |
| FRUCTOSE (CONSTITUENT OF CRANBERRY LIQUID PREPARATION) [DSC] |
| Fructose, Pharmaceutical Secondary Standard; Certified Reference Material |
| D-(-)-Fructose, BioReagent, suitable for cell culture, suitable for insect cell culture |
| 25702-76-5 |
| D-(-)-Fructose, analytical standard, analytical standard for fructose assay kit, for use with enzymatic assay kit FA20 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|