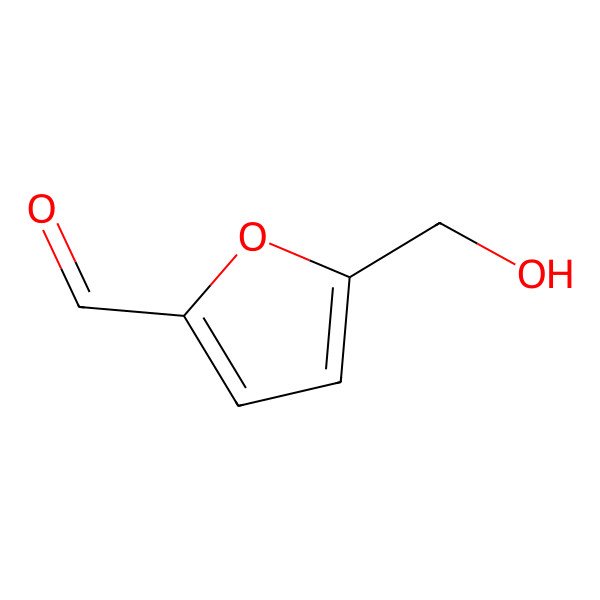
| 67-47-0 |
| 5-Hydroxymethyl-2-furaldehyde |
| 5-(hydroxymethyl)furan-2-carbaldehyde |
| 5-(Hydroxymethyl)furfural |
| 5-(Hydroxymethyl)-2-furaldehyde |
| Hydroxymethylfurfural |
| 5-(Hydroxymethyl)-2-furfural |
| 5-Oxymethylfurfurole |
| 5-Hydroxymethylfuraldehyde |
| Hydroxymethylfurfurole |
| 5-(Hydroxymethyl)-2-furancarbonal |
| 2-Hydroxymethyl-5-furfural |
| 5-Hydroxymethylfuran-2-aldehyde |
| Hydroxymethylfurfuraldehyde |
| 5-Hydroxymethyl-2-formylfuran |
| Hydroxymethylfurfuralaldehyde |
| 5-Hydroxymethyl-2-furfural |
| 2-Furancarboxaldehyde, 5-(hydroxymethyl)- |
| 5-(Hydroxymethyl)-2-furfuraldehyde |
| 5-Hydroxymethylfurfuraldehyde |
| 5-(Hydroxymethyl)-2-furancarboxaldehyde |
| 5-(Hyddroxymethyl)furfurole |
| Aes-103 |
| HMF |
| NSC-40738 |
| 5-Hydroxymethyl-2-furancarboxaldehyde |
| 5-HYDROXYMETHYL-FURFURAL |
| 5-HMF |
| 2-Furaldehyde, 5-(hydroxymethyl)- |
| 5-Hydroxymethyl-2-furfuraldehyde |
| CHEBI:412516 |
| 5-(Hydroxymethyl)furan-2-aldehyde |
| 5-Hydroxymethyl-2-furancarbaldehyde |
| MFCD00003234 |
| NSC 40738 |
| CCRIS 3160 |
| EINECS 200-654-9 |
| 5-(Hydroxymethyl)furfurole |
| Aes 103 |
| BRN 0110889 |
| UNII-70ETD81LF0 |
| 2-formyl-5-hydroxymethylfuran |
| 70ETD81LF0 |
| DTXSID3030428 |
| HSDB 7982 |
| 5-(Hydroxymethyl)-2-formylfuran |
| 5-Hydroxymethyl-furan-2-carbaldehyde |
| 5-hydroxymethyl furfural |
| 5-18-01-00130 (Beilstein Handbook Reference) |
| DTXCID1010428 |
| CAS-67-47-0 |
| 5-methylolfurfural |
| 5-(Hydroxymethyl)furan-2-carboxaldehyde |
| 5-hydromethyl-furfural |
| 5hydroxymethyl_furfural |
| 5-Hydrxoymethylfurfural |
| 5-formylfurfuryl alcohol |
| 5HMF |
| 5-hydroxy methyl furfural |
| Epitope ID:136033 |
| 5-Hydroxymethyl furaldehyde |
| CBiol_000485 |
| SCHEMBL19176 |
| MLS002454379 |
| CHEMBL185885 |
| QSPL 022 |
| GTPL10939 |
| HYDROXYMETHYLFURFURAL, 5- |
| 5-hydroxymethylfuran-2-carbaldehyde |
| BCP31207 |
| CS-D1116 |
| HY-Y0051 |
| NSC40738 |
| Tox21_201892 |
| Tox21_303551 |
| BBL100102 |
| BDBM50487911 |
| GEO-01528 |
| ICCB3_000133 |
| s3772 |
| STL451297 |
| 5-Hydroxymethyl-2-furaldehyde, 99% |
| 5-(hydroxymethyl)-2-furfural (HMF) |
| AKOS005166879 |
| AC-1262 |
| CCG-266098 |
| DB12298 |
| GS-3074 |
| LS-1562 |
| 5-(hydroxymethyl)-furan-2-carbaldehyde |
| NCGC00091513-01 |
| NCGC00091513-02 |
| NCGC00257266-01 |
| NCGC00259441-01 |
| 5-(hydroxymethyl)-2-furan-carboxaldehyde |
| 5-(Hydroxymethyl)furfural, >=99%, FG |
| SMR000393981 |
| SY010869 |
| 5-(hydroxy methyl) 2-furan-carboxaldehyde |
| A9037 |
| AM20100616 |
| FT-0600483 |
| H0269 |
| 5-(HYDROXYMETHYL)-2-FURALDEHYDE [MI] |
| EN300-82764 |
| 5-(Hydroxymethyl)furfural, analytical standard |
| Q414606 |
| Q-200545 |
| 5-hydroxymethylfurfural; 5-(hydroxymethyl)-2-furaldehyde |
| F2191-0155 |
| Z1238542812 |
| 5-(hydroxymethyl)furan-2-carbaldehyde;5-(Hydroxymethyl)furfural |
| InChI=1/C6H6O3/c7-3-5-1-2-6(4-8)9-5/h1-3,8H,4H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|