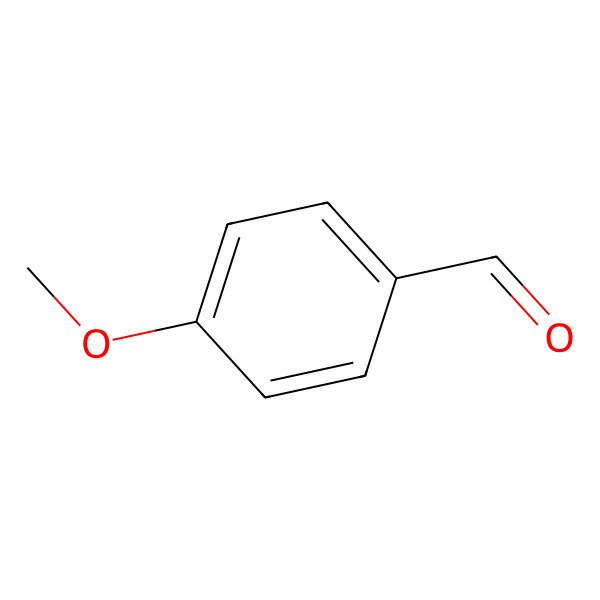
| 123-11-5 |
| P-ANISALDEHYDE |
| Anisic aldehyde |
| Anisaldehyde |
| p-Methoxybenzaldehyde |
| Aubepine |
| 4-Anisaldehyde |
| p-Formylanisole |
| Crategine |
| Obepin |
| p-Anisic aldehyde |
| Benzaldehyde, 4-methoxy- |
| para-anisaldehyde |
| 4-Methoxy-benzaldehyde |
| Formylanisole, p- |
| anisal |
| Caswell No. 051E |
| FEMA No. 2670 |
| NSC 5590 |
| CCRIS 821 |
| 4-methoxy benzaldehyde |
| MFCD00003385 |
| para-methoxybenzaldehyde |
| p-Methoxybenzaldehyde (natural) |
| HSDB 2641 |
| EINECS 204-602-6 |
| UNII-9PA5V6656V |
| p-methoxy benzaldehyde |
| DTXSID2026997 |
| AI3-00223 |
| 9PA5V6656V |
| NSC-5590 |
| p-Methoxybenzaldehyde-13C6 |
| 4-(Methoxy-d3)benzaldehyde |
| CHEMBL161598 |
| DTXCID906997 |
| CHEBI:28235 |
| EC 204-602-6 |
| EINECS 256-891-3 |
| BRN 0471382 |
| 50984-52-6 |
| P-ANISALDEHYDE (USP-RS) |
| P-ANISALDEHYDE [USP-RS] |
| p-Anisaldehyde,p-Methoxybenzaldhyde |
| CAS-123-11-5 |
| MEPYRAMINE MALEATE IMPURITY P (EP IMPURITY) |
| MEPYRAMINE MALEATE IMPURITY P [EP IMPURITY] |
| anisaldehyd |
| Anis aldehyde |
| FEMA 2670 |
| 4-methoxybenzaldehye |
| 4-methoxybezaldehyde |
| Anisaldehyde (para) |
| Anisaldhyde (para-) |
| P-Anisaldehyde,(S) |
| 4-methoxylbenzaldehyde |
| p-Anisaldehyde, 8CI |
| 4-methoxybenzylaldehyde |
| p-Anisaldehyde, 98% |
| p-Anisaldehyde, Reagent |
| benzaldehyde, 4-methoxy |
| para-methoxy benzaldehyde |
| Benzaldehido, 4-metoxi- |
| 4-(methyloxy)benzaldehyde |
| p-Anisaldehyde-%7CA-d1 |
| bmse010130 |
| WLN: VHR DO1 |
| ANISALDEHYDE [INCI] |
| SCHEMBL1100 |
| P-ANISALDEHYDE [MI] |
| 68894-36-0 |
| MLS002152921 |
| NATURAL ANISIC ALDEHYDE |
| P-ANISALDEHYDE [HSDB] |
| p-Methoxybenzylidenemalonitrile |
| 4-methoxybenzene carboxaldehyde |
| p-Methoxy Benzaldehyde, Natural |
| PARA- METHOXYBENZALDEHYDE |
| NSC5590 |
| HMS3039F08 |
| p-Anisaldehyde, analytical standard |
| P-METHOXYBENZALDEHYDE [FCC] |
| HY-Y0740 |
| p-Anisaldehyde, natural, 98%, FG |
| P-METHOXYBENZALDEHYDE [FHFI] |
| Tox21_201943 |
| Tox21_303331 |
| AC7808 |
| BDBM50139370 |
| s5086 |
| STL194068 |
| AKOS000118814 |
| CCG-214805 |
| CS-W020189 |
| LS-2093 |
| Methoxybenzaldehyde; (p-Anisaldehyde) |
| p-Anisaldehyde, for synthesis, 98.0% |
| NCGC00090807-01 |
| NCGC00090807-02 |
| NCGC00257076-01 |
| NCGC00259492-01 |
| p-Anisaldehyde, >=97.5%, FCC, FG |
| AC-10379 |
| CS-11005 |
| LS-20018 |
| SMR001224521 |
| SY001689 |
| A0480 |
| BENZALDEHYDE,4-METHOXY MFC8 H8 O2 |
| FT-0617622 |
| FT-0662227 |
| FT-0662228 |
| EN300-16096 |
| 4-08-00-00252 (Beilstein Handbook Reference) |
| A805017 |
| Q174937 |
| Q-100105 |
| Z53833125 |
| F2190-0575 |
| p-Anisaldehyde, primary pharmaceutical reference standard |
| p-Anisaldehyde, certified reference material, TraceCERT(R) |
| p-Anisaldehyde, United States Pharmacopeia (USP) Reference Standard |
| InChI=1/C8H8O2/c1-10-8-4-2-7(6-9)3-5-8/h2-6H,1H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|