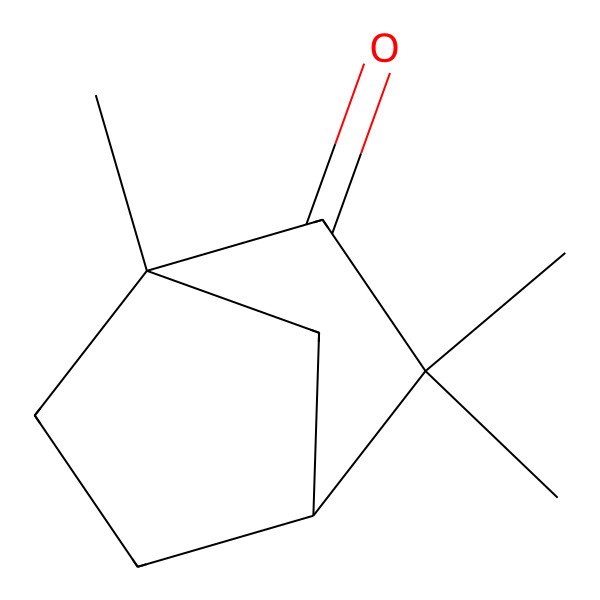
| 1,3,3-Trimethylbicyclo[2.2.1]heptan-2-one |
| 1195-79-5 |
| 214-804-6 |
| CAS-1195-79-5 |
| dl-fenchone |
| EINECS 214-804-6 |
| Fenchone |
| fenchone, (+-)-isomer |
| Fenchon |
| alpha-Fenchone |
| 1,3,3-Trimethyl-2-norbornanone |
| 2-Norbornanone, 1,3,3-trimethyl- |
| 1,3,3-Trimethylnorcamphor |
| 1,3,3-Trimethyl-2-norcamphanone |
| Fenchon [German] |
| NSC 8896 |
| D-FENCHONE, 96 |
| NSC 122687 |
| Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl- |
| CHEBI:4999 |
| 126-21-6 |
| DTXSID9025324 |
| 2-Fenchanone, D- |
| d-Fenchone (natural) |
| BRN 1861492 |
| NCGC00166292-01 |
| 1,3-Trimethylnorcamphor |
| AI3-00736 |
| Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-, (1S,4R)- |
| Bicyclo(2.2.1)heptan-2-one, 1,3,3-trimethyl- |
| 1,3-Trimethyl-2-norbornanone |
| 1,3-Trimethyl-2-norcamphanone |
| 2-Norbornanone,3,3-trimethyl- |
| (+-)-Fenchone |
| 3,3-Dimethyl-8,9-dinorbornan-2-one |
| WLN: L55 A CVTJ B1 D1 D1 |
| 1,3-Trimethylbicyclo[2.2.1]heptan-2-one |
| Bicyclo[2.2.1]heptan-2-one,3,3-trimethyl- |
| UNII-4Q6W8568TG |
| (1R)-1,3,3-Trimethylbicyclo(2.2.1)heptan-2-one |
| (1S)-1,3,3-Trimethylbicyclo[2.2.1]heptan-2-one |
| 1,3,3-Trimethylbicyclo(2.2.1)heptan-2-one, (1S)- |
| Bicyclo(2.2.1)heptan-2-one, 1,3,3-trimethyl-, (1S)- |
| Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-, (1S)- |
| NSC-8896 |
| iso-Fenchone |
| fenchan-2-one |
| NSC-122687 |
| 1,3,3 - trimethylnorbornan - 2 - one |
| 2-07-00-00093 (Beilstein Handbook Reference) |
| .ALPHA.-FENCHONE |
| (1S)-1,3,3-Trimethylbicyclo(2.2.1)heptan-2-one |
| 2-Norbornanone, 1,3,3-trimethyl-, (1R,4S)-()- |
| 2,2,4-trimethylbicyclo[2.2.1]heptan-3-one |
| 2-Norbornanone, 1,3,3-trimethyl-, (1S,4R)-(-)- |
| SCHEMBL57584 |
| (1S) - 1,3,3 - trimethylbicyclo(2.2.1)heptan - 2 - one |
| Bicyclo(2.2.1)heptan-2-one, 1,3,3-trimethyl-, (1S,4R)- |
| DTXCID305324 |
| CHEMBL2268554 |
| NSC8896 |
| AMY25685 |
| Tox21_112396 |
| BBL018824 |
| LS-625 |
| MFCD00248429 |
| NSC122687 |
| s5934 |
| STK802502 |
| AKOS009158536 |
| LMPR0102120016 |
| LS-2759 |
| VS-06784 |
| F0163 |
| F0164 |
| FT-0604551 |
| FT-0627597 |
| 1.3.3-trimethylbicyclo(2.2.1)heptan-2-one |
| EN300-54085 |
| C09859 |
| 1,3,3-Trimethyl-bicyclo[2.2.1]heptan-2-one |
| Bicyclo[2.2.1]heptane-2-one,1,3,3-trimethyl |
| A872224 |
| Q414784 |
| SR-01000945193 |
| 3,3 - dimethyl - 8,9 - dinorbornan - 2 - one |
| SR-01000945193-1 |
| Z804948554 |
| BICYCLO[2.2.1]HEPTAN-2-ONE, 1,3,3,-TRIMETHYL- |
| Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-, (.+/-.)- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|