| 501-97-3 |
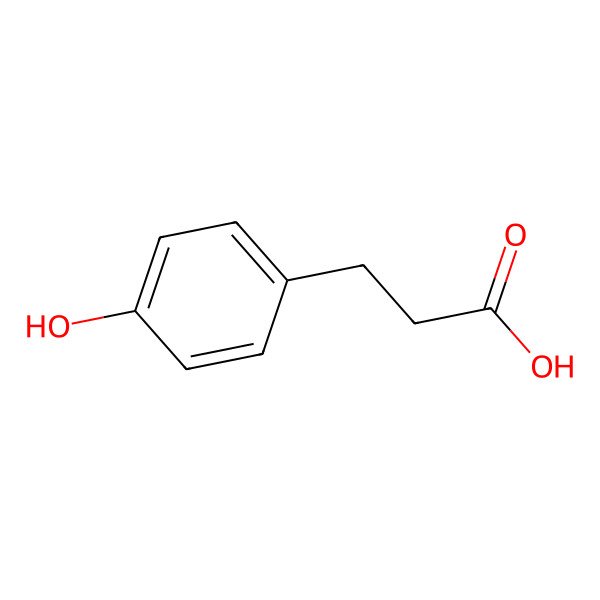
| Phloretic acid |
| 3-(4-Hydroxyphenyl)propanoic acid |
| desaminotyrosine |
| Hydro-p-coumaric acid |
| Benzenepropanoic acid, 4-hydroxy- |
| Dihydro-p-coumaric acid |
| 4-Hydroxyphenylpropionic acid |
| p-Hydroxyhydrocinnamic acid |
| 3-(p-Hydroxyphenyl)propionic acid |
| 4-Hydroxybenzenepropanoic acid |
| p-Hydroxyphenylpropionic acid |
| 4-HYDROXYHYDROCINNAMIC ACID |
| Hydrocinnamic acid, p-hydroxy- |
| beta-(p-Hydroxyphenyl)propionic acid |
| Phloretinic acid |
| HYDROXYPHENYL PROPIONIC ACID |
| uHLORETIC ACID |
| NSC 40949 |
| Dihydrocoumaric acid |
| 3-(4'-Hydroxyphenyl)propionic acid |
| 3-(4-Hydroxyphenyl)-propionic acid |
| EINECS 207-931-3 |
| MFCD00002778 |
| UNII-6QNC6P18SR |
| BRN 2209841 |
| 6QNC6P18SR |
| 4'-Hydroxyphenylpropionic acid |
| AI3-31909 |
| 4-hydroxy-benzenepropanoic acid |
| CHEBI:32980 |
| NSC-40949 |
| 3-(4-hydroxyphenyl)propanoate |
| .beta.-(p-Hydroxyphenyl)propionic acid |
| 4-10-00-00631 (Beilstein Handbook Reference) |
| 3-(p-Hydroxyphenyl)propionate |
| p-hydroxy-hydrocinnamic acid |
| Phloretinate |
| hydro-p-coumarate |
| Dihydro-p-coumarate |
| CADMIUMNITRATE |
| p-Hydroxyhydrocinnamate |
| p-hydroxy-hydrocinnamate |
| p-hydroxyphenylpropionate |
| 3-(p-HYDROXYPHENYL)-PROPIONIC ACID |
| hydroxy-hydrocinnamic acid |
| 4-Hydroxybenzenepropanoate |
| N-hydroxysuccinimide ester |
| 4-Hydroxybenzylacetic Acid |
| 4-hydroxy-benzenepropanoate |
| bmse010064 |
| p-hydroxy-benzene propionate |
| 4-hydroxyphenylpropanoic acid |
| Oprea1_221751 |
| SCHEMBL35486 |
| 4-(4-Hydroxyphenylpropanoate |
| b-(p-Hydroxyphenyl)propionate |
| 3-(4-Hydroxyphenyl)-propionate |
| beta-(p-hydroxyphenyl)propionate |
| CHEMBL1172560 |
| DTXSID2075427 |
| 3-(para-Hydroxyphenyl)propionate |
| 4-(4-Hydroxyphenylpropanoic acid |
| 3-(4'-Hydroxyphenyl)-propionate |
| 3-(4-Hydroxy-phenyl)-propionate |
| 3-(p-hydroxyphenyl)propanoic acid |
| 4-(2-CARBOXYETHYL)PHENOL |
| 4-hydroxy-(9CI)benzenepropanoate |
| b-(p-Hydroxyphenyl)propionic acid |
| BDBM231636 |
| NSC40949 |
| NSC65596 |
| 3(4-hydroxy phenyl) propionic acid |
| 3-(4-hydroxy-phenyl)propanoic acid |
| 3-(4-hydroxy-phenyl)propionic acid |
| 3-(4-hydroxyphenyl)-propanoic acid |
| 3-[4-hydroxy phenyl]propionic acid |
| 2,3-DIHYDRO-P-COUMARIC ACID |
| 3-(para-Hydroxyphenyl)propionic acid |
| BBL012597 |
| NSC-65596 |
| s3795 |
| STK803270 |
| 3-(4 Hydroxy-phenyl)-propionic acid |
| 3-(4'-Hydroxyphenyl)-propionic acid |
| 3-(4-hydroxy phenyl) propionic acid |
| 3-(4-Hydroxy-phenyl)-propanoic acid |
| 3-(4-Hydroxy-phenyl)-propionic acid |
| 4-hydroxy-(9CI)benzenepropanoic acid |
| 3-(para-Hydroxyphenyl)-propionic acid |
| AKOS000193936 |
| AB00269 |
| AC-5144 |
| AT13193 |
| CCG-266325 |
| CS-W016062 |
| DB03897 |
| HY-W015346 |
| propanoic acid, 3-(4-hydroxyphenyl)- |
| PS-5760 |
| P-HYDROXY-BENZENE PROPIONIC ACID |
| NCGC00382803-03 |
| 3-(4-Hydroxyphenyl)propionic acid, 98% |
| LS-77204 |
| 3-(4-HYDROXYPHENYL) PROPIONIC ACID |
| AM20050166 |
| FT-0613674 |
| H0599 |
| EN300-50375 |
| C01744 |
| M03860 |
| AQ-358/40179730 |
| Q-200485 |
| Q7186527 |
| F2191-0066 |
| Z317038012 |
| InChI=1/C9H10O3/c10-8-4-1-7(2-5-8)3-6-9(11)12/h1-2,4-5,10H,3,6H2,(H,11,12 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|