| 50-89-5 |
| deoxythymidine |
| 2'-Deoxythymidine |
| Thymidin |
| 5-Methyldeoxyuridine |
| Beta-Thymidine |
| DThyd |
| 5-Methyl-2'-deoxyuridine |
| Thymine-2-desoxyriboside |
| 5-Methyldeoxyurindine |
| Deoxyribothymidine |
| Thymine-2-deoxyriboside |
| Thyminedeoxyriboside |
| Thymine deoxyriboside |
| dThd |
| 2'-thymidine |
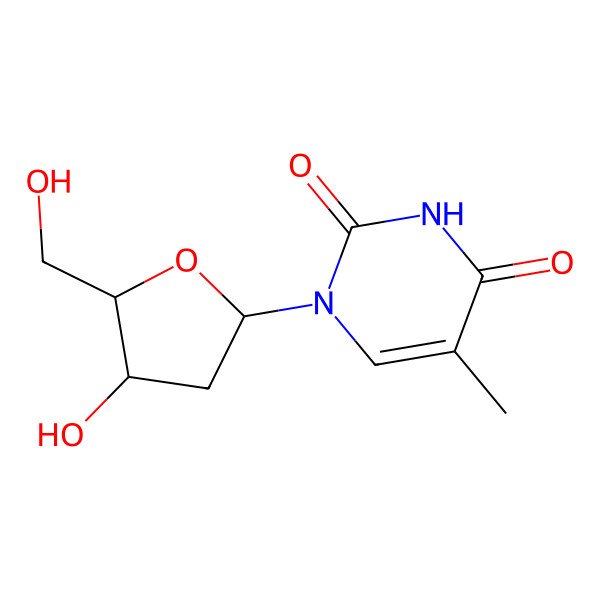
| 1-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione |
| Uridine, 2'-deoxy-5-methyl- |
| dT |
| Doxribtimine |
| 157049-39-3 |
| thymine 2'-deoxyriboside |
| 2'-deoxy-5-methyluridine |
| AI3-52267 |
| beta-D-Ribofuranoside, thymine-1 2-deoxy- |
| NSC 21548 |
| 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione |
| CCRIS 1283 |
| CHEBI:17748 |
| 1-(2-Deoxy-beta-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione |
| 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-beta-D-erythro-pentofuranosyl)-5-methyl- |
| Thymine 2-desoxyriboside |
| EINECS 200-070-4 |
| UNII-VC2W18DGKR |
| VC2W18DGKR |
| L-Thymidine |
| 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione |
| DTXSID5023661 |
| NSC-21548 |
| [3H]-Thymidine |
| NCGC00142484-03 |
| MFCD00006537 |
| alpha-tritiated thymidine |
| EC 200-070-4 |
| 146183-25-7 |
| THM |
| Telbivudine[USAN] |
| 1-(2-deoxy-beta-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione |
| 1-(2-Deoxy-beta-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione (Thymidine) |
| 157049-40-6 |
| 50-88-4 |
| thymidin- |
| Thymidine- |
| deoxyribosylthymine |
| 4qsv |
| 1,2'-deoxy-beta-L-ribofuranosylthymine |
| Thymidinedeoxyriboside |
| beta-Methyldeoxyuridine |
| 1w2g |
| Thymidine, >=99% |
| BDBM1 |
| THYMIDINE [MI] |
| THYMIDINE [INCI] |
| Thymidine (8CI,9CI) |
| DOXRIBTIMINE [INN] |
| bmse000244 |
| D01GLG |
| D0X4JL |
| Epitope ID:138113 |
| THYMIDINE [MART.] |
| DOXRIBTIMINE [USAN] |
| THYMIDINE [WHO-DD] |
| THYMIDINE [WHO-IP] |
| 2'-deoxy-5-methyl-Uridine |
| 1-(2-Deoxy-beta-D-ribofuranosyl)-5-methyluracil |
| SCHEMBL19894 |
| 2'-dT |
| CHEMBL52609 |
| DTXCID703661 |
| GTPL4718 |
| IMPURITY C [EP IMPURITY] |
| 1-(2-Deoxy-beta-L-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-Pyrimidinedione |
| IQFYYKKMVGJFEH-XLPZGREQSA-N |
| HY-N1150 |
| STR05630 |
| Thymidine, >=99.0% (HPLC) |
| Tox21_111560 |
| HG1139 |
| s4803 |
| AKOS015895360 |
| STAVUDINE IMPURITY C [WHO-IP] |
| thymine-1 2-deoxy-b-D-Ribofuranoside |
| AC-1475 |
| AM83949 |
| CCG-266886 |
| CS-W019644 |
| DB04485 |
| CAS-50-89-5 |
| DEOXYTHYMIDINE; 2'-DEOXYTHYMIDINE |
| NCGC00142484-01 |
| 1-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydro-furan-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione |
| 1-[(4S,2R,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,3-dihydropyri midine-2,4-dione |
| BP-58693 |
| STAVUDINE IMPURITY C [EP IMPURITY] |
| Thymidine, Vetec(TM) reagent grade, 99% |
| LS-153777 |
| ZIDOVUDINE IMPURITY E [EP IMPURITY] |
| T0233 |
| thymine-1 2-deoxy-beta-delta-Ribofuranoside |
| MT-1621 COMPONENT 2'-DEOXYTHYMIDINE |
| 1-(2-Deoxy-beta-ribofuranosyl)-5-methyluracil |
| C00214 |
| EN300-378408 |
| A828337 |
| Q422464 |
| SR-01000883981 |
| (1-[2-Deoxy-beta-D-ribofuranosyl]-5-methyluracil) |
| 1-(2-Deoxy-.beta.-D-ribofuranosyl)-5-methyluracil |
| J-700251 |
| SR-01000883981-1 |
| BRD-K28309349-001-02-4 |
| Q27124084 |
| D7E43A69-265B-4DAA-BC8F-193FB357140F |
| Thymidine, powder, BioReagent, suitable for cell culture |
| Z1741976665 |
| 1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione |
| 1-(2-Deoxy-b-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione |
| 1-(2-Deoxy-beta-delta-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione |
| 1-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione |
| 1-(2-DEOXY-.BETA.-D-ERYTHRO-PENTOFURANOSYL)-5-METHYLPYRIMIDINE-2,4(1H,3H)-DIONE [WHO-IP] |
| 1-(2-Deoxy-beta-D-ribofuranosyl)-5-methyluracil; 1-(2-Deoxy-beta-D-ribofuranosyl)thymine; Thymine deoxyriboside; 2'-Deoxythymidine; 5-Methyldeoxyuridine |
| 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione |
| 1211376-53-2 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|