| 145-13-1 |
| Arthenolone |
| 3beta-Hydroxypregn-5-en-20-one |
| Pregnetan |
| Pregneton |
| Pregnolon |
| Prenolon |
| Regnosone |
| Skinostelon |
| Enelone |
| 5-Pregnen-3beta-ol-20-one |
| Natolone |
| Bina-Skin |
| delta5-Pregnenolone |
| 5-Pregnen-3-beta-ol-20-one |
| Pregnenolona |
| Pregnenolonum |
| (3BETA)-3-HYDROXYPREGN-5-EN-20-ONE |
| 5-Pregnenolone |
| Pregnenolone [INN:BAN] |
| 3-beta-Hydroxypregn-5-en-20-one |
| NSC 1616 |
| Pregnenolonum [INN-Latin] |
| Pregnenolona [INN-Spanish] |
| Prestwick_859 |
| 3beta-Hydroxy-5-pregnen-20-one |
| Pregn-5-en-20-one, 3-hydroxy-, (3beta)- |
| Prestwick3_000546 |
| EINECS 205-647-4 |
| Pregnenolone (JAN/INN) |
| Pregn-5-en-20-one, 3beta-hydroxy- |
| UNII-73R90F7MQ8 |
| 3b-Hydroxy-5-pregnen-20-one |
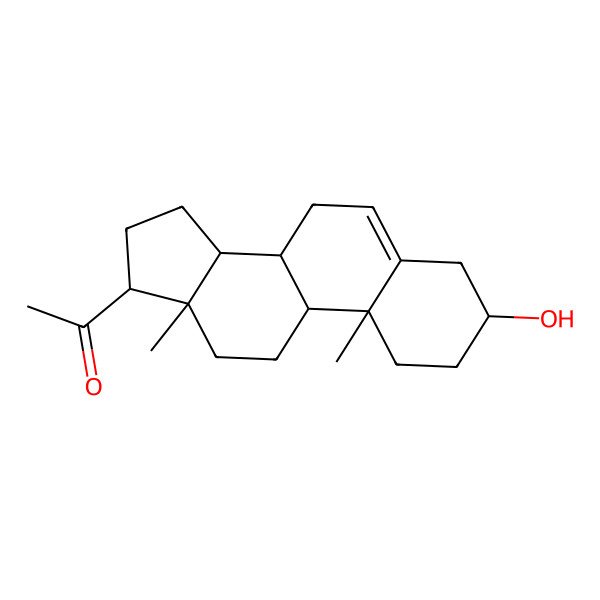
| 1-[(3S,8S,9S,10R,13S,14S,17S)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]ethanone |
| DTXSID1036541 |
| Pregn-5-en-20-one, 3-hydroxy-, (3b)- |
| Pregn-5-ene-3beta-ol-20-one |
| CHEBI:16581 |
| Pregn-5-en-20-one, 3-hydroxy-, (3-beta)- |
| 73R90F7MQ8 |
| 3.beta.-Hydroxypregn-5-en-20-one |
| Pregn-5-en-20-one, 3-beta-hydroxy- |
| 5-Pregnen-3beta;-ol-20-one |
| DTXCID9016541 |
| PLO |
| SMR000112161 |
| .DELTA.5-Pregnenolone |
| Pregnenolone (3beta-Hydroxypregn-5-en-20-one) |
| SR-05000002128 |
| 5-Pregnen-3.beta.-ol-20-one |
| Pregnolone |
| delta-5-pregnen-3beta-ol-20-one |
| NSC-1616 |
| delta-5-pregnen-3-beta-ol-20-one |
| NSC-18158 |
| (3-beta)-3-Hydroxypregn-5-en-20-one |
| NCGC00163125-02 |
| Pregn-5-en-20-one, 3.beta.-hydroxy- |
| CAS-145-13-1 |
| Prestwick0_000546 |
| Prestwick1_000546 |
| Prestwick2_000546 |
| Spectrum5_002057 |
| PREGNENOLONE [MI] |
| PREGNENOLONE [INN] |
| PREGNENOLONE [JAN] |
| bmse000476 |
| D0B4RU |
| Pregnenolone (schizophrenia) |
| 5-Pregnen-3b-ol-20-one |
| PREGNENOLONE [VANDF] |
| BIDD:PXR0019 |
| BSPBio_000591 |
| MLS002153868 |
| MLS002207138 |
| Pregn-5-en-3b-ol-20-one |
| PREGNENOLONE [WHO-DD] |
| SCHEMBL129572 |
| Pregn-5-ene-3b-ol-20-one |
| SPBio_002512 |
| 3-Hydroxy-5-pregnen-20-one |
| 3b-Hydroxypregn-5-en-20-one |
| BPBio1_000651 |
| CHEMBL253363 |
| GTPL2376 |
| Pregn-5-en-3beta-ol-20-one |
| delta5-Pregnen-3beta-ol-20-one |
| 3beta-Hydroxy-5-pregenen-20-one |
| 3beta-Hydroxy-5-pregnene-20-one |
| 3beta-hydroxypregn-5-ene-20-one |
| HMS1569N13 |
| HMS2090J15 |
| HMS2096N13 |
| HMS2268A04 |
| HMS3713N13 |
| HMS3884G16 |
| Delta5-Pregenen-3beta-ol-20-one |
| Delta5-Pregnene-3beta-ol-20-one |
| 1-[(3S,8S,9S,10R,13S,14S,17S)-10,13-Dimethyl-3-hydroxy-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]ethanone |
| BCP23361 |
| HY-B0151 |
| Tox21_112011 |
| Tox21_302141 |
| BDBM50375319 |
| CMC_13393 |
| LMST02030088 |
| Pregnenolone (progesterone precursor) |
| s1914 |
| 3beta-Hydroxy-delta5-pregnen-20-one |
| AKOS015841076 |
| AKOS015895418 |
| Tox21_112011_1 |
| (3b)-3-hydroxy-Pregn-5-en-20-one |
| CCG-220546 |
| CS-1970 |
| DB02789 |
| DS-7794 |
| Pregnenolone 0.1 mg/ml in Acetonitrile |
| .DELTA.5-Pregnen-3.beta.-ol-20-one |
| 5-Pregnen-3beta-ol-20-one, >=98% |
| NCGC00163125-03 |
| NCGC00163125-04 |
| NCGC00163125-06 |
| NCGC00255436-01 |
| AC-12806 |
| AC-32630 |
| BCP0726000217 |
| Neurosteroid (schizophrenia), Neuroscience |
| Pregn-5-n-20-ne, 3-ydroxy-, (3)- |
| Pregnenolone (schizophrenia), Neuroscience |
| LS-118759 |
| 3-Hydroxypregn-5-en-20-one, (3.beta.)- |
| 3.beta.-Hydroxy-.DELTA.5-pregnen-20-one |
| P0786 |
| C01953 |
| D00143 |
| 17beta-[1-Ketoethyl]-Delta5-androsten-3beta-ol |
| EN300-7388669 |
| Pregn-5-en-20-one, 3-hydroxy-, (3.beta.)- |
| Q412158 |
| J-008059 |
| SR-05000002128-4 |
| SR-05000002128-5 |
| .DELTA. SUP(5)-PREGNEN-3.BETA.-OL-20-ONE |
| BRD-K43880410-001-14-4 |
| 0F6367C4-E1FA-4AEC-9846-7BCC5ADA3417 |
| Z2235801892 |
| PREGN-5-EN-20-ONE, 3-(3-CARBOXY-, (3.BETA.)- |
| 17.BETA.-(1-KETOETHYL)-.DELTA. SUP(5)-ANDROSTEN-3.BETA.-OL |
| 1-((3S,8S,10R,13S,17S)-3-Hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-ethanone |
| 1-((3S,8S,9S,10R,13S,14S,17S)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethan-1-one |
| 1-[(1S,3aS,3bS,7S,9aR,9bS,11aS)-7-hydroxy-9a,11a-dimethyl-1H,2H,3H,3aH,3bH,4H,6H,7H,8H,9H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-1-yl]ethan-1-one |
| 1-[(3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16, 17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)]ethanone |
|
There are more than 10 synonyms. If you wish to see them all click here.
|