| 18524-94-2 |
| Loganoside |
| 7-Hydroxy-6-desoxyverbenalin |
| CHEBI:15771 |
| UNII-H7WJ16Q93C |
| H7WJ16Q93C |
| EINECS 242-398-0 |
| C17H26O10 |
| NSC 606403 |
| LOGANIN, (-)- |
| NSC-606403 |
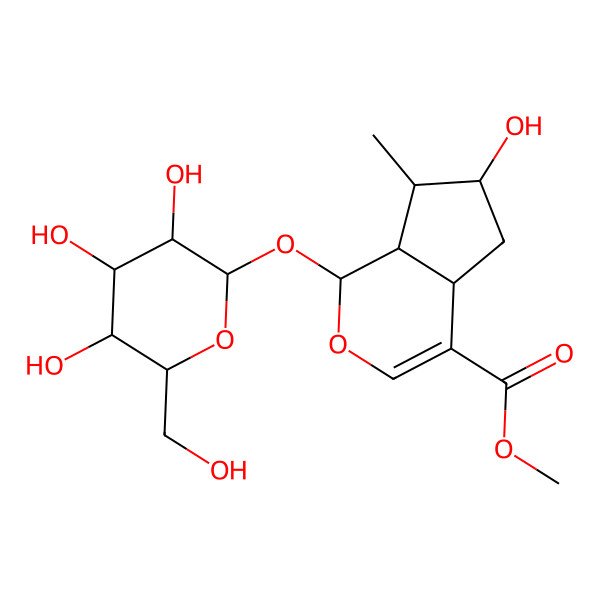
| (1S,4aS,6S,7R,7aS)-Methyl 6-hydroxy-7-methyl-1-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate |
| Cyclopenta(c)pyran-4-carboxylic acid, 1-(beta-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methyl-, methyl ester, (1S-(1alpha,4aalpha,6alpha,7alpha,7aalpha))- |
| methyl (1S,4aS,6S,7R,7aS)-6-hydroxy-7-methyl-1-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4aH,5H,6H,7H,7aH-cyclopenta[c]pyran-4-carboxylate |
| (-)-Loganin |
| Meliatin |
| 1-(beta-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta(c)pyran-4-carboxylic acid methyl ester |
| 1-(beta-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylic acid methyl ester |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1-(.BETA.-D-GLUCOPYRANOSYLOXY)-1,4A,5,6,7,7A-HEXAHYDRO-6-HYDROXY-7-METHYL-, METHYL ESTER, (1S-(1.ALPHA.,4A.ALPHA.,6.ALPHA.,7.ALPHA.,7A.ALPHA.))- |
| Cyclopenta[c]pyran-4-carboxylic acid, 1-(.beta.-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methyl-, methyl ester, [1S-(1.alpha.,4a.alpha.,6.alpha.,7.alpha.,7a.alpha.)]- |
| methyl (1S,4aS,6S,7R,7aS)-1-(beta-D-glucopyranosyloxy)-6-hydroxy-7-methyl-1,4a,5,6,7,7a-hexahydrocyclopenta(c)pyran-4-carboxylate |
| methyl (1S,4aS,6S,7R,7aS)-1-(beta-D-glucopyranosyloxy)-6-hydroxy-7-methyl-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate |
| Methyl (1S-(1alpha,4aalpha,6alpha,7alpha,7aalpha))-1-(beta-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta(c)pyran-4-carboxylate |
| Methyl [1S-(1alpha,4aalpha,6alpha,7alpha,7aalpha)]-1-(beta-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[c]pyran-4-carboxylate |
| Spectrum_001503 |
| SpecPlus_000563 |
| LOGANIN [MI] |
| Spectrum2_001637 |
| Spectrum3_001875 |
| Spectrum4_001914 |
| Spectrum5_000628 |
| Loganin, analytical standard |
| BSPBio_003350 |
| KBioGR_002535 |
| KBioSS_001983 |
| DivK1c_006659 |
| SCHEMBL307017 |
| SPECTRUM1504066 |
| SPBio_001733 |
| MEGxp0_000723 |
| 7-hydroxy-6-nu-desoxyverbenalin |
| CHEMBL1081584 |
| ACon1_001749 |
| KBio1_001603 |
| KBio2_001983 |
| KBio2_004551 |
| KBio2_007119 |
| KBio3_002852 |
| AMBQHHVBBHTQBF-UOUCRYGSSA-N |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1-(.BETA.-D-GLUCOPYRANOSYLOXY)-1,4A,5,6,7,7A-HEXAHYDRO-6-HYDROXY-7-METHYL-, METHYL ESTER |
| HY-N0512 |
| BDBM50279529 |
| CCG-38757 |
| MFCD00075645 |
| s3835 |
| AKOS022190418 |
| CS-5019 |
| LMPR0102070001 |
| SDCCGMLS-0066747.P001 |
| NCGC00178124-01 |
| NCGC00178124-02 |
| NCGC00178124-04 |
| AC-34479 |
| AS-75232 |
| cyclopenta(c)pyran-4-carboxylic acid, 1-(beta-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methyl-, methyl ester, (1S, 4aS, 6S, 7R, 7aS)- |
| methyl (1S,4aS,6S,7R,7aS)-6-hydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate |
| C01433 |
| A812937 |
| Q-100220 |
| 5A5AFFBA-93AB-4635-A071-FC87B9DC023D |
| Q15426222 |
| (1S)-1.ALPHA.-(.BETA.-D-GLUCOPYRANOSYLOXY)-1,4A.ALPHA.,5,6,7,7A.ALPHA.-HEXAHYDRO-6.ALPHA.-HYDROXY-7.ALPHA.-METHYLCYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID METHYL ESTER |
| (1S)-1alpha-(beta-D-GLUCOPYRANOSYLOXY)-1,4Aalpha,5,6,7,7Aalpha-HEXAHYDRO-6alpha-HYDROXY-7alpha-METHYLCYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID METHYL ESTER |
| (1S,4aS,6S,7R,7aS)-methyl 6-hydroxy-7-methyl-1-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate |
| (1S,4aS,6S,7R,7aS)-Methyl6-hydroxy-7-methyl-1-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate |
| 6-hydroxy-7-methyl-1-[[3,4,5-trihydroxy-6-(hydroxymethyl)-2-oxanyl]oxy]-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylic acid methyl ester |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1-(.BETA.-D-GLUCOPYRANOSYLOXY)-1,4A,5,6,7,7A-HEXAHYDRO-6-HYDROXY-7-METHYL-, METHYL ESTER, (1S,4AS,6S,7R,7AS)- |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1-(beta-D-GLUCOPYRANOSYLOXY)-1,4A,5,6,7,7A-HEXAHYDRO-6-HYDROXY-7-METHYL-, METHYL ESTER |
| CYCLOPENTA(C)PYRAN-4-CARBOXYLIC ACID, 1-(beta-D-GLUCOPYRANOSYLOXY)-1,4A,5,6,7,7A-HEXAHYDRO-6-HYDROXY-7-METHYL-, METHYL ESTER, (1S,4AS,6S,7R,7AS)- |
| Cyclopenta[c]pyran-4-carboxylic acid,1-(b-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methyl-, methyl ester, (1S,4aS,6S,7R,7aS)- |
| methyl (1S,4aS,6S,7R,7aS)-6-hydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate |
| METHYL (1S-(1.ALPHA.,4A.ALPHA.,6.ALPHA.,7.ALPHA.,7A.ALPHA.))-1-(.BETA.-D-GLUCOPYRANOSYLOXY)-1,4A,5,6,7,7A-HEXAHYDRO-6-HYDROXY-7-METHYLCYCLOPENTA(C)PYRAN-4-CARBOXYLATE |
| Methyl 6-hydroxy-7-methyl-1-((3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,4a,5 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|