| 1180-71-8 |
| Dictamnolactone |
| Obaculactone |
| Citrolimonin |
| Evodin |
| Limonine |
| 7,16-Dioxo-7,16-dideoxylimondiol |
| Limoni |
| Limonoic acid 3,19:16,17 dilactone |
| Limonoic acid, di-delta-lactone |
| limonoic acid di-delta-lactone |
| NSC 36508 |
| limonoic acid 3,19:16,17-dilactone |
| Evodia fruit |
| Limonoate D-ring-lactone |
| CCRIS 4047 |
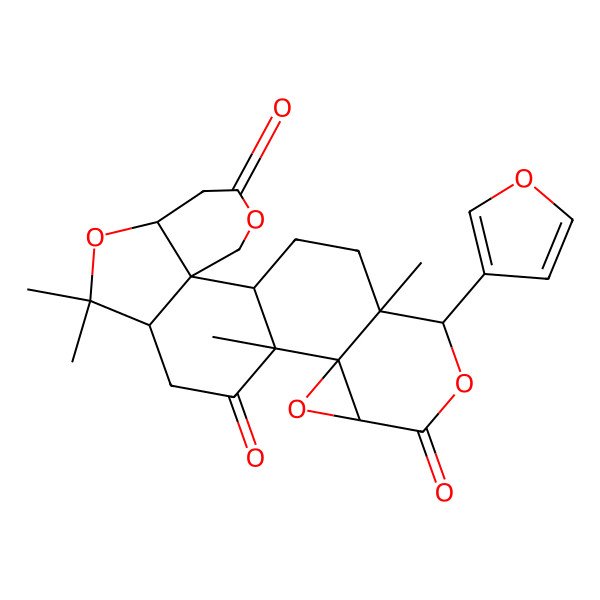
| (4aS,6aR,8aR,8bR,9aS,12S,12aS,14aR,14bR)-12-(furan-3-yl)-6,6,8a,12a-tetramethyldecahydro-1H,3H-oxireno[2,3-d]pyrano[4',3':3,3a]isobenzofuro[5,4-f]isochromene-3,8,10(6H,9aH)-trione |
| DTXSID8045985 |
| UNII-L0F260866S |
| CHEBI:16226 |
| Limonoic acid, di-.delta.-lactone |
| L0F260866S |
| AI3-37932 |
| (4aS,6aR,8aR,8bR,9aS,12S,12aS,14aR,14bR)-12-(Furan-3-yl)-6,6,8a,12a-tetramethyldecahydrooxireno[2,3-d]pyrano[4',3':3,3a]isobenzofuro[5,4-f]isochromene-3,8,10(1H,6H,8aH)-trione |
| 11H,13H-Oxireno(d)pyrano(4',3':3,3a)isobenzofuro(5,4-f)(2)benzopyran-4,6,13(2H,5aH)-trione, 8-(3-furyl)decahydro-2,2,4a,8a-tetramethyl- |
| DTXCID6025985 |
| Limone |
| MFCD00075922 |
| (1R,2R,7S,10R,13R,14R,16S,19S,20S)-19-(furan-3-yl)-9,9,13,20-tetramethyl-4,8,15,18-tetraoxahexacyclo[11.9.0.02,7.02,10.014,16.014,20]docosane-5,12,17-trione |
| (4aS,6aR,8aR,8bR,9aS,12R,12aS,14aR,14bR)-12-(3-furyl)-6,6,8a,12a-tetramethyldecahydro-3H-oxireno[d]pyrano[4',3':3,3a][2]benzofuro[5,4-f]isochromene-3,8,10(6H,9aH)-trione |
| 11H,13H-Oxireno(d)pyrano(4',3':3,3a)isobenzofuro(5,4-f)(2)benzopyran-4,6,13(2H,5aH)-trione, 8-(3-furanyl)decahydro-2,2,4a,8a-tetramethyl-, (2aR,4aR,4bR,5aS,8S,8aS,10aR,10bR,14aS)- |
| 1809582-56-6 |
| rel-(4aS,6aR,8aR,8bR,9aS,12S,12aS,14aR,14bR)-12-(Furan-3-yl)-6,6,8a,12a-tetramethyldecahydrooxireno[2,3-d]pyrano[4',3':3,3a]isobenzofuro[5,4-f]isochromene-3,8,10(1H,6H,8aH)-trione |
| CAS-1180-71-8 |
| 3-furyl(tetramethyl)[?]trione |
| Linonin |
| NSC36508 |
| NSC-36508 |
| NCGC00095714-01 |
| 11H,13H-Oxireno[d]pyrano[4',3':3,3a]isobenzofuro[5,4-f][2]benzopyran-4,6,13(2H,5aH)-trione, 8-(3-furyl)decahydro-2,2,4a,8a-tetramethyl- |
| LIMONIN [MI] |
| Spectrum2_001728 |
| Spectrum3_001012 |
| Spectrum4_001140 |
| Spectrum5_000935 |
| Limonin, analytical standard |
| BSPBio_002763 |
| KBioGR_001659 |
| Limonoic acid di-del.-lactone |
| SCHEMBL320315 |
| SPECTRUM1800018 |
| SPBio_001776 |
| CHEMBL517449 |
| ACon1_001996 |
| KBio3_001983 |
| Limonoic acid di-.delta.-lactone |
| KBDSLGBFQAGHBE-MSGMIQHVSA-N |
| 11H,13H-Oxireno(d)pyrano(4',3':3,3a)isobenzofuro(5,4-f)(2)benzopyran-4,6,13(2H,5aH)-trione, 8-(3-furanyl)decahydro-2,2,4a,8a-tetramethyl-, (2aR-(2aalpha,4abeta,4bR,5aalpha,8alpha,8aalpha,10aalpha,10bR*,14aalpha))- |
| Tox21_111513 |
| BDBM50418089 |
| CCG-38796 |
| AKOS015965307 |
| Tox21_111513_1 |
| AC-8045 |
| CS-1237 |
| SDCCGMLS-0066837.P001 |
| NCGC00178483-01 |
| NCGC00178483-02 |
| NCGC00178483-04 |
| NCGC00178483-05 |
| NCGC00178483-14 |
| NCGC00263659-01 |
| AS-15249 |
| HY-17411 |
| L0258 |
| Limonin, from citrus seeds, >90% (HPLC) |
| S2319 |
| C03514 |
| A854522 |
| Q-100083 |
| Q2398745 |
| BRD-K05906022-001-03-6 |
| BRD-K05906022-001-05-1 |
| Limone; Citrolimonin; Evodine; Dictamnolactone; Obaculactone |
| (4aS,14bR)-12-(Furan-3-yl)-6,6,8a,12a-tetramethyldecahydro-1H,3H-oxireno[2,3-d]pyrano[4',3':3,3a]isobenzofuro[5,4-f]isochromene-3,8,10(6H,9aH)-trione |
| (4aS,6aR,8aR,8bR,9aS,12R,12aS,14aR,14bR)-12-(furan-3-yl)-6,6,8a,12a-tetramethyldecahydro-3H-oxireno[d]pyrano[4',3':3,3a][2]benzofuro[5,4-f]isochromene-3,8,10(6H,9aH)-trione |
|
There are more than 10 synonyms. If you wish to see them all click here.
|