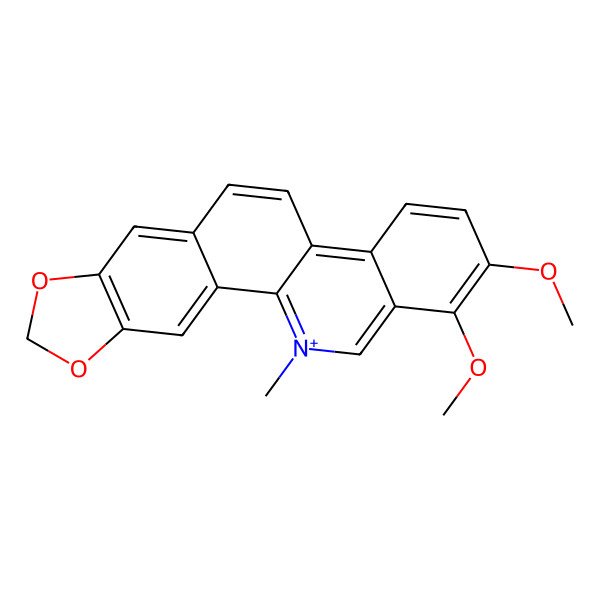
Chelerythrine
| Internal ID | 336d2507-9f8b-4ded-b21a-3b72dc9b339a |
| Taxonomy | Alkaloids and derivatives > Benzophenanthridine alkaloids > Quaternary benzophenanthridine alkaloids |
| IUPAC Name | 1,2-dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridin-12-ium |
| SMILES (Canonical) | C[N+]1=C2C(=C3C=CC(=C(C3=C1)OC)OC)C=CC4=CC5=C(C=C42)OCO5 |
| SMILES (Isomeric) | C[N+]1=C2C(=C3C=CC(=C(C3=C1)OC)OC)C=CC4=CC5=C(C=C42)OCO5 |
| InChI | InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 |
| InChI Key | LLEJIEBFSOEYIV-UHFFFAOYSA-N |
| Popularity | 1,841 references in papers |
| Molecular Formula | C21H18NO4+ |
| Molecular Weight | 348.40 g/mol |
| Exact Mass | 348.12358306 g/mol |
| Topological Polar Surface Area (TPSA) | 40.80 Ų |
| XlogP | 4.60 |
| 34316-15-9 |
| Toddalin |
| cheleritrine |
| broussonpapyrine |
| Toddaline |
| EINECS 251-930-0 |
| [1,3]Benzodioxolo[5,6-c]phenanthridinium, 1,2-dimethoxy-12-methyl- |
| UNII-E3B045W6X0 |
| CHEBI:78373 |
| 1,2-dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridin-12-ium |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| CHEMBL1293255 | P15428 | 15-hydroxyprostaglandin dehydrogenase [NAD+] |
19952.6 nM |
Potency |
via CMAUP
|
| CHEMBL3577 | P00352 | Aldehyde dehydrogenase 1A1 |
22387.2 nM 15848.9 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL3687 | P18054 | Arachidonate 12-lipoxygenase |
12589.3 nM |
Potency |
via CMAUP
|
| CHEMBL2903 | P16050 | Arachidonate 15-lipoxygenase |
19952.6 nM |
Potency |
via CMAUP
|
| CHEMBL4096 | P04637 | Cellular tumor antigen p53 |
12589.3 nM 5011.9 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL3356 | P05177 | Cytochrome P450 1A2 |
10000 nM |
AC50 |
via CMAUP
|
| CHEMBL3622 | P33261 | Cytochrome P450 2C19 |
10000 nM |
Potency |
via CMAUP
|
| CHEMBL3397 | P11712 | Cytochrome P450 2C9 |
39810.7 nM |
Potency |
via CMAUP
|
| CHEMBL289 | P10635 | Cytochrome P450 2D6 |
25118.86 nM |
AC50 |
via CMAUP
|
| CHEMBL340 | P08684 | Cytochrome P450 3A4 |
31622.8 nM 31622.8 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL4159 | Q99714 | Endoplasmic reticulum-associated amyloid beta-peptide-binding protein |
25118.9 nM 19952.6 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha |
31622.8 nM 31622.8 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL4040 | P28482 | MAP kinase ERK2 |
31622.8 nM |
Potency |
via CMAUP
|
| CHEMBL1293224 | P10636 | Microtubule-associated protein tau |
15848.9 nM 15848.9 nM 14125.4 nM 17782.8 nM 501.2 nM |
Potency Potency Potency Potency Potency |
via CMAUP
via CMAUP via CMAUP via CMAUP via Super-PRED |
| CHEMBL1951 | P21397 | Monoamine oxidase A |
220 nM |
Ki |
via Super-PRED
|
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit |
11220.2 nM 11220.2 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL235 | P37231 | Peroxisome proliferator-activated receptor gamma |
566 nM |
IC50 |
via Super-PRED
|
| CHEMBL1293294 | P51151 | Ras-related protein Rab-9A |
46109.1 nM |
Potency |
via CMAUP
|
| CHEMBL2842 | P42345 | Serine/threonine-protein kinase mTOR |
2930.9 nM |
Potency |
via CMAUP
|
| CHEMBL1293232 | Q16637 | Survival motor neuron protein |
7079.5 nM |
Potency |
via CMAUP
|
| CHEMBL1293256 | P40225 | Thrombopoietin |
1995.3 nM 1995.3 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL1963 | P16473 | Thyroid stimulating hormone receptor |
25118.9 nM 25118.9 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL1293227 | O75604 | Ubiquitin carboxyl-terminal hydrolase 2 |
25118.9 nM |
Potency |
via CMAUP
|
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL4303 | P08238 | Heat shock protein HSP 90-beta | 96.59% | 96.77% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 92.93% | 94.45% |
| CHEMBL2635 | P51452 | Dual specificity protein phosphatase 3 | 90.18% | 94.00% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 89.96% | 91.11% |
| CHEMBL225 | P28335 | Serotonin 2c (5-HT2c) receptor | 89.79% | 89.62% |
| CHEMBL2373 | P21730 | C5a anaphylatoxin chemotactic receptor | 88.86% | 92.62% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 88.62% | 95.56% |
| CHEMBL4481 | P35228 | Nitric oxide synthase, inducible | 88.58% | 94.80% |
| CHEMBL1293249 | Q13887 | Kruppel-like factor 5 | 88.28% | 86.33% |
| CHEMBL1075094 | Q16236 | Nuclear factor erythroid 2-related factor 2 | 88.17% | 96.00% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 84.63% | 96.09% |
| CHEMBL5925 | P22413 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 | 84.36% | 92.38% |
| CHEMBL3713062 | P10646 | Tissue factor pathway inhibitor | 83.97% | 97.33% |
| CHEMBL2535 | P11166 | Glucose transporter | 83.92% | 98.75% |
| CHEMBL1937 | Q92769 | Histone deacetylase 2 | 82.40% | 94.75% |
| CHEMBL4306 | P22460 | Voltage-gated potassium channel subunit Kv1.5 | 80.95% | 94.03% |
| CHEMBL5747 | Q92793 | CREB-binding protein | 80.58% | 95.12% |
| CHEMBL5608 | Q16288 | NT-3 growth factor receptor | 80.12% | 95.89% |
| CHEMBL215 | P09917 | Arachidonate 5-lipoxygenase | 80.09% | 92.68% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| PubChem | 2703 |
| NPASS | NPC100079 |
| ChEMBL | CHEMBL13045 |
| LOTUS | LTS0231770 |
| wikiData | Q5089853 |