| 54-28-4 |
| D-gamma-Tocopherol |
| (+)-gamma-Tocopherol |
| 7,8-Dimethyltocol |
| o-Xylotocopherol |
| Gamma tocopherol |
| .gamma.-Tocopherol |
| RRR-gamma-Tocopherol |
| gamma-Tocopherol, d- |
| Rrr-.gamma.-tocopherol |
| (r,r,r)-.gamma.-tocopherol |
| UNII-8EF1Z1238F |
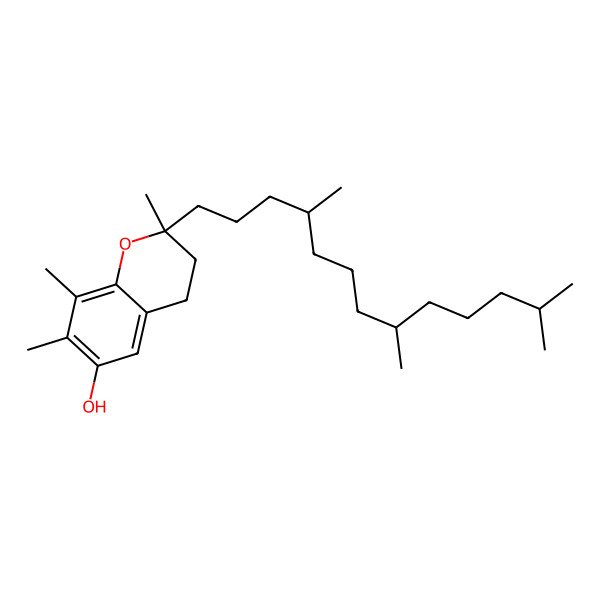
| (2R)-2,7,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol |
| DTXSID9049031 |
| CHEBI:18185 |
| 8EF1Z1238F |
| EINECS 200-201-5 |
| NCGC00185764-01 |
| J213.540J |
| (R)-2,7,8-trimethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)chroman-6-ol |
| (2R)-2,7,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-chromen-6-ol |
| (+)-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol |
| (R,R,R)-gamma-Tocopherol |
| (2R)-3,4-dihydro-2,7,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-2H-1-benzopyran-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-, (2R)- |
| Vitamin E succinate (calcium) |
| 7,8-Dimethyltocolo-xylotocopherol |
| tocopherol gamma |
| Vitamin E gamma |
| gamma -Tocopherol |
| ??-Tocopherol |
| 3,4-Dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-ol |
| (2R(2R*(4R*,8R*)))-3,4-Dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzopyran-6-ol |
| (2R)-3,4-DIHYDRO-2,7,8-TRIMETHYL-2-((4R,8R)-4,8,12-TRIMETHYLTRIDECYL)-2H-1-BENZOPYRAN-6-OL |
| [2R[2R*(4R*,8R*)]]-3,4-dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzopyran-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)-, (2R)- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-, (2R-(2R*(4R*,8R*)))- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-, [2R-[2R*(4R*,8R*)]]- |
| (+)-g-Tocopherol |
| (+)-y-Tocopherol |
| (+)-.gamma.-Tocopherol |
| SCHEMBL120346 |
| CHEMBL2151591 |
| DTXCID0028957 |
| D--Tocopherol;(+)--Tocopherol |
| .GAMMA.-TOCOPHEROL [MI] |
| GAMMA-TOCOPHEROL [WHO-DD] |
| HY-N7148 |
| Tox21_113559 |
| LMPR02020065 |
| AKOS040759356 |
| DB15394 |
| CAS-54-28-4 |
| NCGC00185764-03 |
| BS-48945 |
| (+)-gamma-Tocopherol, >=96% (HPLC) |
| (+)-gamma-Tocopherol, analytical standard |
| CS-0103047 |
| C02483 |
| F75916 |
| Q155753 |
| RRR-.ALPHA.-TOCOPHEROL IMPURITY C [EP IMPURITY] |
| 2,7,8-Trimethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol |
| DL-alpha-Tocopherol succinate calcium;-Tocopherol succinate calcium |
| (2R)-3,4-Dihydro-2,7,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltri-decyl]-2H-1-benzopyran-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,7,8-trimethyl- 2-(4,8,12-trimethyltridecyl)-, [2R-[2R(4R,8R)]]- |
| 2H-1-Benzopyran-6-ol,3,4-dihydro-2,7,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-, (2R)- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|