cis-Resveratrol
| Internal ID | 6e53fe2a-ca93-4830-927b-2673e9785d1f |
| Taxonomy | Phenylpropanoids and polyketides > Stilbenes |
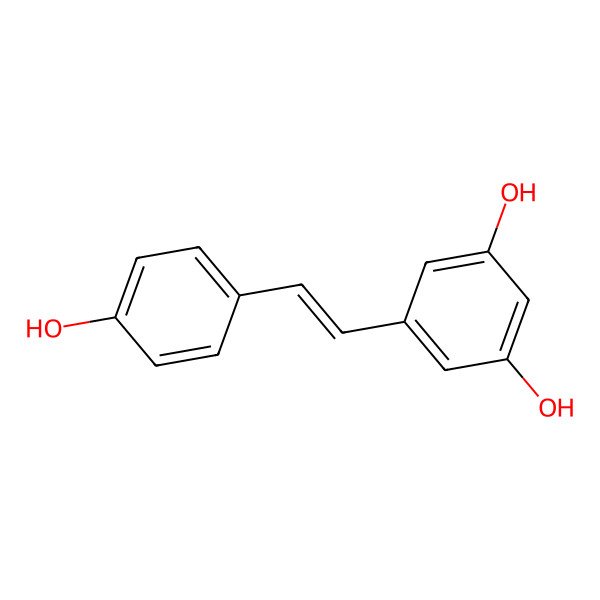
| IUPAC Name | 5-[(Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol |
| SMILES (Canonical) | C1=CC(=CC=C1C=CC2=CC(=CC(=C2)O)O)O |
| SMILES (Isomeric) | C1=CC(=CC=C1/C=C\C2=CC(=CC(=C2)O)O)O |
| InChI | InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1- |
| InChI Key | LUKBXSAWLPMMSZ-UPHRSURJSA-N |
| Popularity | 11,725 references in papers |
| Molecular Formula | C14H12O3 |
| Molecular Weight | 228.24 g/mol |
| Exact Mass | 228.078644241 g/mol |
| Topological Polar Surface Area (TPSA) | 60.70 Ų |
| XlogP | 3.10 |
| (Z)-resveratrol |
| 61434-67-1 |
| Cis resveratrol |
| Resveratrol, (Z)- |
| 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol |
| (Z)-3,5,4'-trihydroxystilbene |
| cis-3,4',5-trihydroxystilbene |
| 5-[(Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol |
| Resveratrol (Z)-form [MI] |
| UNII-AUA0K06FSB |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| CHEMBL1293255 | P15428 | 15-hydroxyprostaglandin dehydrogenase [NAD+] |
15848.9 nM 35481.3 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL3577 | P00352 | Aldehyde dehydrogenase 1A1 |
8912.5 nM 39810.7 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL2903 | P16050 | Arachidonate 15-lipoxygenase |
19952.6 nM |
Potency |
via CMAUP
|
| CHEMBL3594 | Q16790 | Carbonic anhydrase IX |
810 nM |
Ki |
via Super-PRED
|
| CHEMBL3242 | O43570 | Carbonic anhydrase XII |
950 nM |
Ki |
via Super-PRED
|
| CHEMBL3510 | Q9ULX7 | Carbonic anhydrase XIV |
830 nM |
Ki |
via Super-PRED
|
| CHEMBL4096 | P04637 | Cellular tumor antigen p53 |
7943.3 nM 7943.3 nM 7943.3 nM |
Potency Potency Potency |
via CMAUP
via CMAUP via CMAUP |
| CHEMBL3356 | P05177 | Cytochrome P450 1A2 |
630.96 nM 199.53 nM |
AC50 AC50 |
via CMAUP
via CMAUP |
| CHEMBL3622 | P33261 | Cytochrome P450 2C19 |
2.5 nM 31622.8 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL3397 | P11712 | Cytochrome P450 2C9 |
25118.9 nM 10000 nM 31622.8 nM |
Potency Potency Potency |
via CMAUP
via CMAUP via CMAUP |
| CHEMBL289 | P10635 | Cytochrome P450 2D6 |
10000 nM 39810.7 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL340 | P08684 | Cytochrome P450 3A4 |
2511.9 nM 3162.3 nM 2511.9 nM 3162.3 nM |
Potency Potency Potency Potency |
via CMAUP
via CMAUP via CMAUP via CMAUP |
| CHEMBL284 | P27487 | Dipeptidyl peptidase IV |
0.6 nM |
IC50 |
via Super-PRED
|
| CHEMBL4159 | Q99714 | Endoplasmic reticulum-associated amyloid beta-peptide-binding protein |
12589.3 nM 12589.3 nM 12589.3 nM 12589.3 nM |
Potency Potency Potency Potency |
via CMAUP
via CMAUP via CMAUP via CMAUP |
| CHEMBL206 | P03372 | Estrogen receptor alpha |
785 nM |
Ki |
via Super-PRED
|
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha |
3162.3 nM 12589.3 nM 3162.3 nM 12589.3 nM |
Potency Potency Potency Potency |
via CMAUP
via CMAUP via CMAUP via CMAUP |
| CHEMBL1293226 | B2RXH2 | Lysine-specific demethylase 4D-like |
12589.3 nM 28183.8 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL1293224 | P10636 | Microtubule-associated protein tau |
14125.4 nM 14125.4 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL4005 | P42336 | PI3-kinase p110-alpha subunit |
250 nM |
IC50 |
via Super-PRED
|
| CHEMBL3145 | P42338 | PI3-kinase p110-beta subunit |
500 nM |
IC50 |
via Super-PRED
|
| CHEMBL1293235 | P02545 | Prelamin-A/C |
4466.8 nM |
Potency |
via CMAUP
|
| CHEMBL1075189 | P14618 | Pyruvate kinase isozymes M1/M2 |
3162.3 nM 3162.3 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL3959 | P16083 | Quinone reductase 2 |
54 nM |
Kd |
via Super-PRED
|
| CHEMBL1293232 | Q16637 | Survival motor neuron protein |
14125.4 nM |
Potency |
via CMAUP
|
| CHEMBL1293256 | P40225 | Thrombopoietin |
12589.3 nM 12589.3 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL3194 | P02766 | Transthyretin |
470 nM |
Kd |
via Super-PRED
|
| CHEMBL1075138 | Q9NUW8 | Tyrosyl-DNA phosphodiesterase 1 |
1 nM 1 nM |
Potency Potency |
via Super-PRED
via CMAUP |
| CHEMBL1293227 | O75604 | Ubiquitin carboxyl-terminal hydrolase 2 |
10000 nM |
Potency |
via CMAUP
|
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 95.52% | 91.11% |
| CHEMBL242 | Q92731 | Estrogen receptor beta | 92.49% | 98.35% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 88.38% | 95.56% |
| CHEMBL1075094 | Q16236 | Nuclear factor erythroid 2-related factor 2 | 87.44% | 96.00% |
| CHEMBL1293249 | Q13887 | Kruppel-like factor 5 | 86.66% | 86.33% |
| CHEMBL2288 | Q13526 | Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 | 84.13% | 91.71% |
| CHEMBL1951 | P21397 | Monoamine oxidase A | 83.75% | 91.49% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 81.12% | 94.45% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| PubChem | 1548910 |
| NPASS | NPC248573 |
| ChEMBL | CHEMBL87333 |
| LOTUS | LTS0195285 |
| wikiData | Q27116664 |