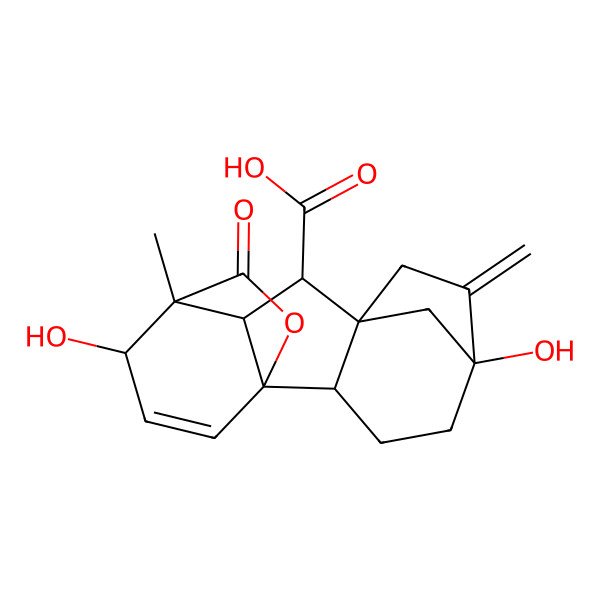
| Gibberellin A3 |
| 77-06-5 |
| Gibberellin |
| Berelex |
| Brellin |
| Gibberellin X |
| Gib-Tabs |
| Gib-Sol |
| Gibberellic acid GA3 |
| Cekugib |
| Gibreskol |
| Grocel |
| Pro-Gibb |
| Pro-Gibb Plus |
| Gibberellins A4A7 |
| Gibrescol |
| Gibefol |
| Regulex |
| Ryzup |
| Gibberellinsaeure |
| Activol GA |
| Pgr-iv |
| Acide gibberellique |
| Gibberellins |
| Activol |
| (+)-Gibberellic Acid |
| GA3 |
| Caswell No. 467 |
| Gibbrel |
| Gibberelic acid |
| CHEBI:28833 |
| gibberellin 3 |
| UNII-BU0A7MWB6L |
| BU0A7MWB6L |
| NCI-C55823 |
| CCRIS 4820 |
| HSDB 712 |
| AI3-52922 |
| Acide gibberellique [ISO-French] |
| EINECS 201-001-0 |
| MFCD00079329 |
| NSC 14190 |
| NSC-14190 |
| Gibb-3-ene-1,10-dicarboxylic acid, 2,4a,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1alpha,2beta,4aalpha,4bbeta,10beta)- |
| EPA Pesticide Chemical Code 043801 |
| BRN 0054346 |
| GA [Plant Growth Regulator] |
| Gibberellic acid [BSI:ISO] |
| Gibberellic acid [ISO:BSI] |
| DTXCID40656 |
| DTXSID0020656 |
| 5-18-09-00269 (Beilstein Handbook Reference) |
| 2,4a,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylic acid 1,4a-lactone |
| Gibb-3-ene-1,10-dicarboxylic acid, 2,4a,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone |
| (1alpha,2beta,4aalpha,4bbeta,10beta)-2,4a,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylic acid 1,4a-lactone |
| (3S,3aR,4S,4aS,7S,9aR,9bR,12S)-7,12-Dihydroxy-3-methyl-6-methylene-2-oxoperhydro-4a,7-methano-9b,3-propenoazuleno(1,2-b)furan-4-carboxylic acid |
| (3S,3aS,4S,4aS,6S,8aR,8bR,11S)-6,11-dihydroxy-3-methyl-12-methylene-2-oxo-4a,6-ethano-3,8b-prop-1-enoperhydroindeno(1,2-b)furan-4-carboxylic acid |
| (3S,3aS,4S,4aS,6S,8aS,8bS,11S)-6,11-Dihydroxy-3-methyl-12-methylene-2-oxo-4a,6-ethano-3,8b-prop-1-enoperhydroindeno(1,2-b)furan-4-carboxylic acid |
| (3S,3aS,4S,4aS,7S,9aR,9bR,12S)-7,12-Dihydroxy-3-methyl-6-methylene-2-oxoperhydro-4a,7-methano-9b,3-propeno(1,2-b)furan-4-carboxylic acid |
| 2beta,4aalpha,7-Trihydroxy-1beta-methyl-8-methylene-4aalpha,4bbeta-gibb-3-ene-1alpha,10beta-dicarboxylic acid 1,4a-lactone |
| 2beta,4alpha,7-Trihydroxy-1-methyl-8-methylene-4aalpha,4bbeta-gibb-3-ene-1alpha,10beta-dicarboxylic acid 1,4a-lactone |
| 2beta,4alpha,7-Trihydroxy-1-methylene-4aalpha,4bbeta-gibb-3-ene-1alpha,10beta-dicarboxylic acid 1,4a-lactone |
| NCGC00091033-01 |
| Gibberellic acid 100 microg/mL in Acetonitrile |
| Gibberellate |
| (3S,3aS,4S,4aS,6S,8aR,8bR,11S)-6,11-Dihydroxy-3-methyl-12-methylene-2-oxo-4a,6-ethano-3,8b-prop-1-enoperhydroindeno[1,2-b]furan-4-carboxylic acid |
| 2,4alpha,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10 beta-dicarboxylic acid 1,4alpha-lactone |
| GA (Plant Growth Regulator) |
| GIBBERELLIC ACID (USP-RS) |
| GIBBERELLIC ACID [USP-RS] |
| C19H22O6 |
| GA3 gibberellin |
| GIBERELLIN |
| 2beta,7alpha-dihydroxy-1beta-methyl-8-methylidene-13-oxo-4a,1alpha-epoxymethano-4aalpha,4bbeta-gibb-3-ene-10beta-carboxylic acid |
| GA(3) gibberellin |
| Gibberelate |
| Gibberelin |
| Gibberillate |
| Ralex |
| NSC14190 |
| NSC19450 |
| Gibberillic acid |
| Gibb-tabs |
| Pro-Vide |
| Release LC |
| 4psb |
| 2,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-carboxylic acid 1-4-lactone |
| CAS-77-06-5 |
| Gibberellin (GA) |
| (1R,2R,5S,8S,9S,10R,11S,12S)-5,12-dihydroxy-11-methyl-6-methylidene-16-oxo-15-oxapentacyclo(9.3.2.1(5,8).0(1,10).0(2,8))heptadec-13-ene-9-carboxylic acid |
| (1R,2R,5S,8S,9S,10R,11S,12S)-5,12-dihydroxy-11-methyl-6-methylidene-16-oxo-15-oxapentacyclo[9.3.2.1(5,8).0(1,10).0(2,8)]heptadec-13-ene-9-carboxylic acid |
| Gibb-3-ene-1, 2,4a,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1.alpha.,2.beta.,4a.alpha.,4b.beta.,10.beta.)- |
| Gibb-3-ene-1,10-dicarboxylic acid, 2,4a,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1.alpha.,2.beta.,4a.alpha.,4b.beta.,10.beta.)- |
| 10365-11-4 |
| GIBERILLIC ACID |
| (+)-Gibberellin A3 |
| Spectrum_000628 |
| 4q0k |
| SpecPlus_000148 |
| 2b-Hydroxygibberellin 1 |
| GibberellinsA currencyure |
| PS49_SUPELCO |
| Prestwick0_000965 |
| Prestwick1_000965 |
| Prestwick2_000965 |
| Prestwick3_000965 |
| Spectrum2_000311 |
| Spectrum3_001301 |
| Spectrum4_001444 |
| Spectrum5_000027 |
| gibberellic acid (ga-3) |
| bmse000317 |
| SCHEMBL15577 |
| BSPBio_000969 |
| BSPBio_002961 |
| KBioGR_001927 |
| KBioSS_001108 |
| MLS001055447 |
| MLS002154076 |
| DivK1c_006244 |
| G7645_SIGMA |
| GIBBERELLIC ACID [MI] |
| SPBio_000302 |
| SPBio_002890 |
| GIBBERELLIC ACID [ISO] |
| BPBio1_001067 |
| MEGxm0_000440 |
| GIBBERELLIC ACID [HSDB] |
| GIBBERELLIC ACID [INCI] |
| GIBBERELLIC ACID, 90% |
| CHEMBL1232952 |
| ACon0_000224 |
| ACon1_000551 |
| BCBcMAP01_000012 |
| KBio1_001188 |
| KBio2_001108 |
| KBio2_003676 |
| KBio2_006244 |
| KBio3_002181 |
| HMS1571A11 |
| HMS2098A11 |
| HMS2231J16 |
| HMS3039M06 |
| (1S,2S,4aR,4bR,7S,9aS,10S,10aR)-2,7-dihydroxy-1-methyl-8-methylene-13-oxo-1,2,4b,5,6,7,8,9,10,10a-decahydro-4a,1-(epoxymethano)-7,9a-methanobenzo[a]azulene-10-carboxylic acid |
| HY-N1964 |
| Tox21_202052 |
| Tox21_303023 |
| BDBM50561592 |
| GEO-04261 |
| MD-920 |
| NSC-19450 |
| s4766 |
| AKOS025310145 |
| CCG-208472 |
| DB07814 |
| SMP1_000136 |
| USEPA/OPP Pesticide Code: 043801 |
| NCGC00091033-02 |
| NCGC00091033-03 |
| NCGC00091033-04 |
| NCGC00091033-05 |
| NCGC00091033-06 |
| NCGC00091033-07 |
| NCGC00091033-09 |
| NCGC00256446-01 |
| NCGC00259601-01 |
| (1R,2R,5S,8S,9S,10R,11S,12S)-5,12-Dihydroxy-11-methyl-6-methylene-16-oxo-15-oxapentacyclo[9.3.2.15,8.01,10.02,8]heptadec-13-ene-9-carboxylic acid |
| AS-14216 |
| NCI60_000922 |
| SMR000686070 |
| SMR001233386 |
| AB00513979 |
| CS-0018282 |
| Gibberellin, 80% gibberellin A3 basis (TLC) |
| Gibberellic acid 1000 microg/mL in Acetonitrile |
| EN300-19631964 |
| Q411138 |
| TU 64-3-103-75 |
| Gibberellic acid, 90% gibberellin A3 basis (HPLC) |
| Gibberellic acid, PESTANAL(R), analytical standard |
| BRD-K92758126-001-05-5 |
| BRD-K92758126-001-06-3 |
| BRD-K92758126-001-17-0 |
| 6F94D8A8-3230-4AB5-93C1-46F5E84FE343 |
| Gibberellic acid, United States Pharmacopeia (USP) Reference Standard |
| 2,4A,7-TRIHYDROXY-1-METHYL-8-METHYLENEGIBB-3-ENE-1,10-CARBOXYLIC ACID 1,4-LACTONE |
| 2,4a,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-carboxylic acid 1-4-lactone |
| Gibberellic Acid, Pharmaceutical Secondary Standard; Certified Reference Material |
| (1.ALPHA.,2.BETA.,4A.ALPHA.,4B.BETA.,10.BETA.)-2,4A,7-TRIHYDROXY-1-METHYL-8-METHYLENEGIBB-3-ENE-1,10-DICARBOXYLIC ACID 1,4A-LACTONE |
| (1R,2R,5S,8S,9S,10R,11S,12S)-5,12-dihydroxy-11-methyl-6-methylidene-16-oxo-15-oxapentacyclo[9.3.2.1^{5,8}.0^{1,10}.0^{2,8}]heptadec-13-ene-9-carboxylic acid |
| (3S,3aR,4S,4aS,7S,9aR,9bR,12S)-7,12-Dihydroxy-3-methyl-6-methylene-2-oxoperhydro-4a,7-methano-9b,3-propenoazuleno |
| (3S,3aS,4S,4aS,6S,8aS,8bS,11S)-6,11-Dihydroxy-3-methyl-12-methylene-2-oxo-4a,6-ethano-3,8b-prop-1-enoperhydroindeno |
| (3S,3aS,4S,4aS,7S,9aR,9bR,12S)-7,12-Dihydroxy-3-methyl-6-methylene-2-oxoperhydro-4a,7-methano-9b,3-propeno |
| 2,4 alpha,7-trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10 beta-dicarboxylic acid 1,4 alpha-lactone |
| 2,4.ALPHA.,7-TRIHYDROXY-1-METHYL-8-METHYLENEGIBB-3-ENE-1,10-DICARBOXYLIC ACID 1,4-.ALPHA.-LACTONE |
| 2,4alpha,7-TRIHYDROXY-1-METHYL-8-METHYLENEGIBB-3-ENE-1,10-DICARBOXYLIC ACID 1,4-alpha-LACTONE |
| 2Beta,4aAlpha,7-trihydroxy-1Beta-methyl-8-methylene-4aAlpha,4bBeta-gibb-3-ene-1alpha,10Beta-dicarboxylic acid 1-4a lactone |
| 2Beta,4Alpha,7-Trihydroxy-1-methyl-8-methylene-4aAlpha,4bBeta-gibb-3-ene-1alpha, 10beta-dicarboxylic acid 1,4a-lactone |
| 3S,3aS,4S, 4aS,6S,8aR,8bR,11S)-6,11-dihydroxy-3-methyl-12-methylene-2-oxo-4a,6-ethano-3, 8b-prop-l-enoperhydroindeno-(1,2-b)furan-4-carboxylic acid |
| 4A,1-(EPOXYMETHANO)-7,9A-METHANOBENZ(A)AZULENE, GIBB-3-ENE-1,10-DICARBOXYLIC ACID DERIV. |
| Gibberellic acid, plant cell culture tested, BioReagent, >=90% gibberellin A3 basis (of total gibberellins.) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|