| 57-87-4 |
| Provitamin D2 |
| Ergosterin |
| Ergosta-5,7,22-trien-3-ol, (3b,22E)- |
| UNII-Z30RAY509F |
| CCRIS 7220 |
| HSDB 395 |
| Z30RAY509F |
| CHEBI:16933 |
| 24-Methylcholesta-5,7,22-trien-3beta-ol |
| Ergosta-5,7,22-trien-3beta-ol |
| Ergosta-5,7,22E-trien-3beta-ol |
| EINECS 200-352-7 |
| Ergosta-5,7,22-trien-3-ol, (3beta,22E)- |
| 24alpha-Methyl-22E-dehydrocholesterol |
| (22E)-ergosta-5,7,22-trien-3beta-ol |
| (22E,24S)-24-methylcholesta-5,7,22-trien-3beta-ol |
| AI3-18876 |
| 24R-Methylcholesta-5,7,2E-trien-3beta-ol |
| DTXSID90878679 |
| ergosta-5:6,7:8,22:23-trien-3-ol |
| (22E)-Ergosta-5,7,22-trien-3.beta.-ol |
| (3beta,22E)-Ergosta-5,7,22-trien-3-ol |
| ERGOSTEROL (USP-RS) |
| ERGOSTEROL [USP-RS] |
| Ergosta-5,7,22-trien-3-ol, (3.beta.,22E)- |
| Provitamin D 2 |
| Pro Vitamin D2 |
| Pro-Vitamin D2 |
| D2, Pro-Vitamin |
| ERGOCALCIFEROL IMPURITY B (EP IMPURITY) |
| ERGOCALCIFEROL IMPURITY B [EP IMPURITY] |
| (3.beta.,22E)-Ergosta-5,7,22-trien-3-ol |
| ergosta-5,7,22-trien-3-ol |
| ergosterol- |
| NSC62791 |
| Provitamine D2 |
| 5,7,22-Ergostatrien-3beta-ol |
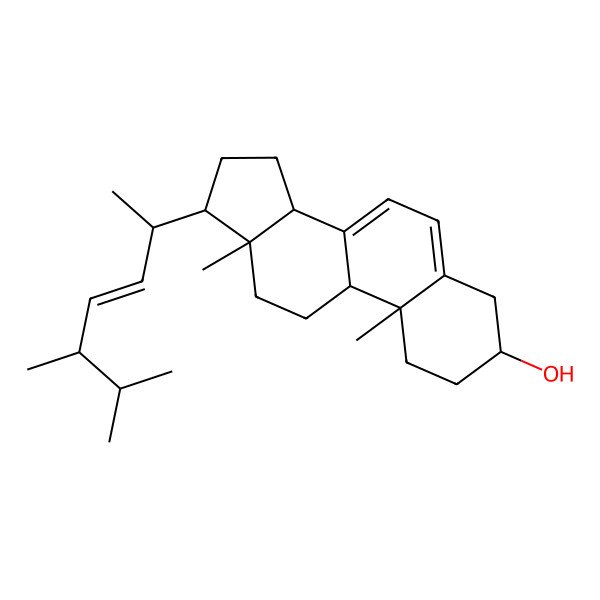
| (3S,9S,10R,13R,14R,17R)-10,13-dimethyl-17-[(E,1R,4R)-1,4,5-trimethylhex-2-enyl]-2,3,4,9,11,12,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol |
| (3S,9S,10R,13R,14R,17R)-17-[(E,2R,5R)-5,6-dimethylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,9,11,12,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol |
| 3beta-Hydroxy-5,7,22-ergostatriene |
| Ergosterol, >=75% |
| ERGOSTEROL [MI] |
| ERGOSTEROL [HSDB] |
| ERGOSTEROL [INCI] |
| bmse000494 |
| Ergosterol (Provitamin D2) |
| SCHEMBL43194 |
| MEGxm0_000450 |
| CHEMBL1232562 |
| ACon0_000429 |
| ACon1_000637 |
| DNVPQKQSNYMLRS-APGDWVJJSA-N |
| DTXCID201016721 |
| 24a-Methyl-22E-dehydrocholesterol |
| Ergosterol, >=95.0% (HPLC) |
| HY-N0181 |
| BDBM50378884 |
| LMST01030093 |
| s2297 |
| 24alpha-Methyl-22E-dehydrocholestero |
| AKOS015918128 |
| AC-8370 |
| DB04038 |
| DS-4956 |
| (24R)-Ergosta-5,7,22-trien-3b-ol |
| (3beta)-Ergosta-5,7,22-trien-3-ol |
| NCGC00168889-01 |
| NCGC00168889-02 |
| (3S,9S,10R,13R,14R,17R)-17-((2R,5R,E)-5,6-dimethylhept-3-en-2-yl)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| 14-((2E)(1R,4R)-1,4,5-trimethylhex-2-enyl)(1S,5S,2R,11R,14R,15R)-2,15-dimethyl tetracyclo[8.7.0.0<2,7>.0<11,15>]heptadeca-7,9-dien-5-ol |
| 24-Methylcholesta-5,7,22-trien-3b-ol |
| 24R-Methylcholesta-5,7,22E-trien-3b-ol |
| (3beta,2E)-Ergosta-5,7,22-trien-3-ol |
| CS-0007890 |
| 24R-Methylcholesta-5,7,22E-trien-3beta-ol |
| DELTA-5,7,22-ERGOSTATRIEN-3BETA-OL |
| (3-beta,22E)-Ergosta-5,7,22-trien-3-ol |
| C01694 |
| Ergosta-5,7,22-trien-3-ol,(3|A,22E)- |
| EN300-21680273 |
| Q143263 |
| 45ED0A4C-6FDA-443F-B886-D6C805A76AF2 |
| Ergosterol, 10 mg/mL in chloroform, analytical standard |
| Ergosterol, European Pharmacopoeia (EP) Reference Standard |
| Ergosterol, United States Pharmacopeia (USP) Reference Standard |
| ERGOSTEROL (CONSTITUENT OF GANODERMA LUCIDUM FRUITING BODY) |
| ERGOSTEROL (CONSTITUENT OF GANODERMA LUCIDUM FRUITING BODY) [DSC] |
| Ergosterol, Pharmaceutical Secondary Standard; Certified Reference Material |
| (1R,3aR,7S,9aR,9bS,11aR)-1-[(2R,3E,5R)-5,6-dimethylhept-3-en-2-yl]-9a,11a-dimethyl-1H,2H,3H,3aH,6H,7H,8H,9H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-7-ol |
|
There are more than 10 synonyms. If you wish to see them all click here.
|