| Melitose |
| D-Raffinose |
| Gossypose |
| Melitriose |
| d-(+)-Raffinose |
| 512-69-6 |
| 6G-alpha-D-galactosylsucrose |
| rafinose |
| raflinose |
| Raffinose, pure |
| NSC 2025 |
| UNII-N5O3QU595M |
| NSC 170228 |
| N5O3QU595M |
| CHEBI:16634 |
| AI3-19427 |
| EINECS 208-146-9 |
| Caryophyllene alcohol acetate |
| beta-D-fructofuranosyl alpha-D-galactopyranosyl-(1->6)-alpha-D-glucopyranoside |
| DTXSID8041111 |
| NSC-2025 |
| NSC-170228 |
| Raffinose hydrate |
| alpha-D-glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1->6)- |
| alpha-D-Glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1-6)- |
| alpha-D-Galp-(1->6)-alpha-D-Glcp-(1->2)-beta-D-Fruf |
| alpha-D-galactopyranosyl-(1->6)-alpha-D-glucopyranosyl beta-D-fructofuranoside |
| alpha-D-galactopyranosyl-(1->6)-alpha-D-glucopyranosyl-(1->2)-beta-D-fructofuranoside |
| alpha-D-Glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl (1 to 6)-, hydrate |
| beta-D-Fructofuranosyl-O-alpha-D-galactopyranosyl-(1->6)-alpha-D-glucopyranoside |
| alpha-D-Glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1-6)-, pentahydrate |
| delta-Raffinose |
| NSC170228 |
| (2S,3R,4S,5S,6R)-2-(((2R,3R,4S,5R,6R)-6-((2S,3S,4R,5S)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl)oxy-3,4,5-trihydroxy-oxan-2-yl)methoxy)-6-(hydroxymethyl)oxane-3,4,5-triol |
| (2S,3R,4S,5S,6R)-2-[[(2R,3R,4S,5R,6R)-6-[(2S,3S,4R,5S)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-3,4,5-trihydroxy-oxan-2-yl]methoxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
| RAF |
| Raffinose (8CI) |
| Melitriose, Raffinose |
| delta-(+)-Raffinose |
| RAFFINOSE [MI] |
| RAFFINOSE [INCI] |
| bmse000221 |
| RAFFINOSE [WHO-DD] |
| SCHEMBL33743 |
| CHEMBL603717 |
| 6G-alpha-delta-galactosylsucrose |
| DTXCID6021111 |
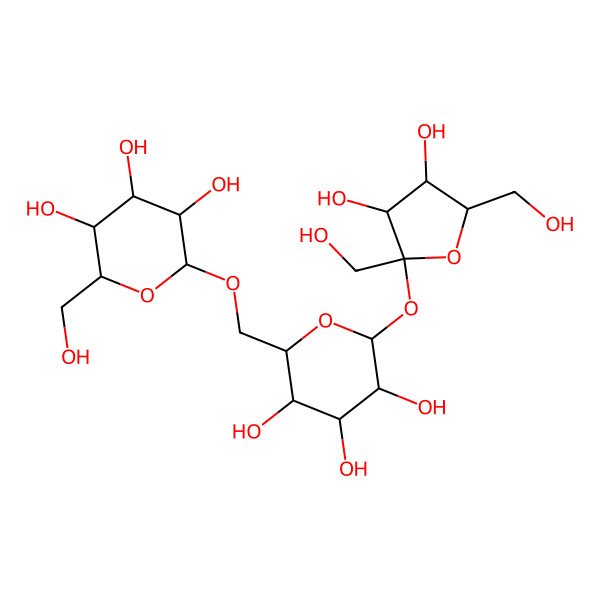
| O(C1OC(COC2OC(CO)C(O)C(O)C2O)C(O)C(O)C1O)C3(CO)OC(CO)C(O)C3O |
| beta-D-Fructofuranosyl-O-alpha-D-galactopyranosyl-(1?6)alpha-D-glucopyranoside |
| HY-N7088 |
| s5155 |
| AKOS025393299 |
| BS-9703 |
| CCG-269725 |
| (2S,3R,4S,5R,6R)-2-[[(2R,3S,4S,5R,6R)-6-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methoxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
| CS-0081719 |
| R0002 |
| C00492 |
| Q410005 |
| 720EEA6C-2455-46E5-9CFA-A6800C4E0D6F |
| alpha-D-Galp-(1->6)-alpha-D-Glcp-(12)-beta-D-Fruf |
| alpha-D-Galp-(1->6)-alpha-D-Glcp-(1<->2)-beta-D-Fruf |
| .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl O-.alpha.-D-galactopyranosyl-(1->6)- |
| alpha-D-Glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl (1 to 6)- |
| alpha-D-Glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1.fwdarw.6)- |
| alpha-D-Glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1>6)- (9CI) |
| O-alp.-D-Galactopyranosyl-(1-->6)-alp.-D-glucopyranosyl-bet.-D-fructofuranoside |
| O-alpha-D-Galactopyranosyl(1,6)-O-alpha-D-glucopyranosyl-(1,2)-beta-D-fructofuranoside |
| (2R,3R,4S,5S,6R)-2-((2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yloxy)-6-(((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro-2H-pyran-3,4,5-triol |
| (2R,3R,4S,5S,6R)-2-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-({[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxane-3,4,5-triol |
| (2S,3R,4S,5R,6R)-2-[[(2R,3S,4S,5R,6R)-6-[(2S,3S,4S,5R)-2,5-bis(hydroxymethyl)-3,4-bis(oxidanyl)oxolan-2-yl]oxy-3,4,5-tris(oxidanyl)oxan-2-yl]methoxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
| (2S,3R,4S,5R,6R)-2-[[(2R,3S,4S,5R,6R)-6-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yl]oxy-3,4,5-trihydroxy-tetrahydropyran-2-yl]methoxy]-6-(hydroxymethyl)tetrahydropyran-3,4,5-triol |
| (2S,3R,4S,5R,6R)-2-[[(2R,3S,4S,5R,6R)-6-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-dimethylol-tetrahydrofuran-2-yl]oxy-3,4,5-trihydroxy-tetrahydropyran-2-yl]methoxy]-6-methylol-tetrahydropyran-3,4,5-triol |
| .BETA.-D-FRUCTOFURANOSYL-O-.ALPHA.-D-GALACTOPYRANOSYL-(1->6)-.ALPHA.-D-GLUCOPYRANOSIDE |
|
There are more than 10 synonyms. If you wish to see them all click here.
|