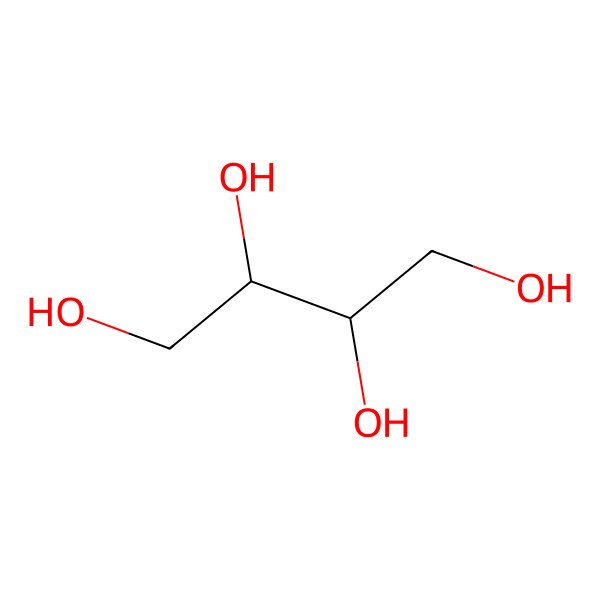
| meso-Erythritol |
| 149-32-6 |
| Phycitol |
| Erythrit |
| Mesoerythritol |
| Phycite |
| Erythrite |
| Erythrol |
| L-Erythritol |
| Antierythrite |
| (2R,3S)-butane-1,2,3,4-tetrol |
| erythro-tetritol |
| Butanetetrol |
| Erythroglucin |
| 1,2,3,4-Butanetetrol, (2R,3S)-rel- |
| i-Erythritol |
| Tetrahydroxybutane |
| Paycite |
| CHEBI:17113 |
| C*Eridex |
| C4H10O4 |
| NIK 242 |
| Cargill Zerose 16957 |
| Erythritol, meso- |
| Erythritol [NF] |
| 1,2,3,4-Butanetetrol |
| (2S,3R)-butane-1,2,3,4-tetrol |
| UNII-RA96B954X6 |
| CCRIS 7901 |
| DTXSID6043919 |
| HSDB 7968 |
| meso-1,2,3,4-Tetrahydroxybutane |
| RA96B954X6 |
| NSC 8099 |
| NSC-8099 |
| EINECS 205-737-3 |
| Erythritol,meso-erythritol |
| ZEROSE TM 16957 |
| INS NO.968 |
| 10030-58-7 |
| DTXCID4023919 |
| FEMA NO. 4819 |
| INS-968 |
| NSC8099 |
| Erythritol (NF) |
| F 8015 |
| Erythrol (VAN) |
| (2r,3s)-butane-1,2,3,4-tetraol |
| 1,2,3,4-Butanetetrol, (R*,S*)- |
| (2R,3S)-rel-1,2,3,4-Butanetetrol |
| rel-(2R,3S)-butane-1,2,3,4-tetraol |
| E-968 |
| ERYTHRITOL (MART.) |
| ERYTHRITOL [MART.] |
| BUTANE-1,2,3,4-TETROL, (2R,3S)- |
| ERYTHRITOL (USP-RS) |
| ERYTHRITOL [USP-RS] |
| BUTANE 1,2,3,4-TETROL (MESO-ERYTHRITOL) |
| Lichen sugar |
| (2R,3S)-rel-Butane-1,2,3,4-tetraol |
| ERYTHRITOL (EP IMPURITY) |
| ERYTHRITOL [EP IMPURITY] |
| ERYTHRITOL (EP MONOGRAPH) |
| ERYTHRITOL [EP MONOGRAPH] |
| 1,2,3,4-Butanetetrol, (theta,S)- |
| MRY |
| SMR000112220 |
| MFCD00004710 |
| meso-Eythritol |
| Lakanto-S |
| Erythritol 100M |
| Erythritol (8CI) |
| L-(-)-Threitol |
| D-ERYTHRITOL |
| ERYTHRITOL [MI] |
| ERYTHRITOL [FCC] |
| ERYTHRITOL [INCI] |
| C* Eridex 16954 |
| WLN: Q1YQYQ1Q |
| ERYTHRITOL [VANDF] |
| 1,3,4-Tetrahydroxybutane |
| Epitope ID:114707 |
| meso-Erythritol, >=99% |
| SCHEMBL17062 |
| MLS001332365 |
| MLS001332366 |
| CHEMBL349605 |
| HMS2270M08 |
| Pharmakon1600-01301025 |
| meso-Erythritol, analytical standard |
| Tox21_200564 |
| NSC760400 |
| s4224 |
| 1,2,3,4-Butanetetrol, (R,S)- |
| 1,3,4-Butanetetrol, (R*,S*)- |
| AKOS006339851 |
| 1 2 3 4-Butanetetrol (R* S*)- |
| AM83963 |
| CCG-266079 |
| DB04481 |
| DS-5851 |
| NSC-760400 |
| NCGC00247033-01 |
| NCGC00258118-01 |
| CAS-149-32-6 |
| E968 |
| 1,2,3,4-butanotetrol, (2r,3s)-rel- |
| E0021 |
| SW219107-1 |
| C00503 |
| D08915 |
| E70403 |
| WURCS=2.0/1,1,0/[h22h]/1/ |
| EN300-1273040 |
| Q421873 |
| F0001-2636 |
| Z1203161930 |
| BDF1567C-B08B-425A-B87F-15FF46328423 |
| Erythritol, European Pharmacopoeia (EP) Reference Standard |
| Erythritol, United States Pharmacopeia (USP) Reference Standard |
| Erythritol, Pharmaceutical Secondary Standard; Certified Reference Material |
|
There are more than 10 synonyms. If you wish to see them all click here.
|