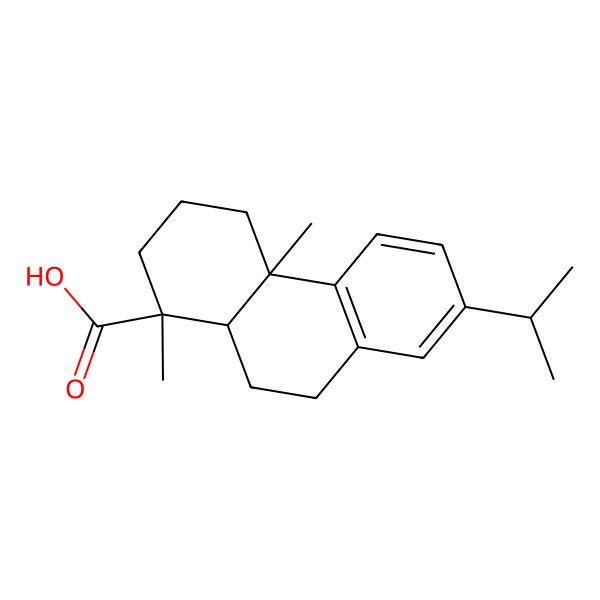
| 1740-19-8 |
| Dehydroabietate |
| (+)-Dehydroabietic acid |
| Abieta-8,11,13-trien-18-oic acid |
| Abietic acid, dehydro- |
| (-)-Dehydroabietic acid |
| 13-Isopropylpodocarpa-8,11,13-trien-15-oic acid |
| NSC 2952 |
| 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, (1R,4aS,10aR)- |
| CHEBI:29571 |
| EINECS 217-102-8 |
| UNII-0S5XP6S3AU |
| BRN 2059290 |
| 0S5XP6S3AU |
| Podocarpa-8,11,13-trien-15-oic acid, 13-isopropyl- |
| NSC2952 |
| Isopropyl podocarpa-8,11,13-trien-15-oic acid |
| NSC-2952 |
| abieta-8(14),9(11),12-trien-18-oic acid |
| 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, (1R-(1alpha,4abeta,10aalpha))- |
| 4-09-00-02389 (Beilstein Handbook Reference) |
| (1R-(1alpha,4Abeta,10aalpha))-1,2,3,4,4a,9,10,10a-octahydro-7-isopropyl-1,4a-dimethylphenanthren-1-carboxylic acid |
| 6980-63-8 |
| (1R,4aS,10aR)-1,4a-dimethyl-7-propan-2-yl-2,3,4,9,10,10a-hexahydrophenanthrene-1-carboxylic acid |
| [1R-(1alpha,4abeta,10aalpha)]-1,2,3,4,4a,9,10,10a-octahydro-7-isopropyl-1,4a-dimethylphenanthren-1-carboxylic acid |
| RESIGRAL 52 |
| CHEMBL12850 |
| SCHEMBL222078 |
| DTXSID8022163 |
| NFWKVWVWBFBAOV-MISYRCLQSA-N |
| Abieta-8,13-trien-18-oic acid |
| 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, [1R-(1.alpha.,4a.beta.,10a.alpha.)]- |
| AMY22483 |
| HY-N6869 |
| BDBM50143600 |
| MFCD09839012 |
| AKOS015917291 |
| CS-W012130 |
| LMPR0104050005 |
| AC-34629 |
| LS-117899 |
| Dehydroabietic acid, >=95% (LC/MS-ELSD) |
| FT-0633812 |
| S3226 |
| 13-Isopropylpodocarpa-8,13-trien-15-oic acid |
| EN300-6730478 |
| A881721 |
| Podocarpa-8,13-trien-15-oic acid, 13-isopropyl- |
| W-107855 |
| Q27110153 |
| 5-Podocarpa-8,11,13-trien-15-oic acid, 13-isopropyl- |
| (1R,4aS,10aR)-1,4a-dimethyl-7-(propan-2-yl)-1,2,3,4,4a,9,10,10a-octahydrophenanthrene-1-carboxylic acid |
| (1R-(1ALPHA,4ABETA,10AALPHA))-1,2,3,4,4A,9,10,10A-OCTAHYDRO- 7-ISOPROPYL-1,4A-DIMETHYLPHENANTHREN-1-CARBOXYLIC ACID |
| 1,2,3,4,4a,9,10,10a-Octahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid |
| 1,3,4,4a,9,10,10a-Octahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid |
| 1-PHENANTHRENECARBOXYLIC ACID, 1,2,3,4,4A,9,10,10A- OCTAHYDRO-1,4A-DIMETHYL-7-(1-METHYLETHYL)-, (1R,4AS,10AR)- |
| 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, [1R-(1,4a,10a)]- |
| 1-Phenanthrenecarboxylic acid,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, [1R-(1.alpha.,4a.beta.,10a.alpha.)]- |
| InChI=1/C20H28O2/c1-13(2)14-6-8-16-15(12-14)7-9-17-19(16,3)10-5-11-20(17,4)18(21)22/h6,8,12-13,17H,5,7,9-11H2,1-4H3,(H,21,22)/t17-,19-,20-/m1/s |
| rel-(1R,4aS,10aR)-7-Isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthrene-1-carboxylic acid |
| rel-(1R,4aS,10aR)-7-Isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthrene-1-carboxylicacid |
|
There are more than 10 synonyms. If you wish to see them all click here.
|