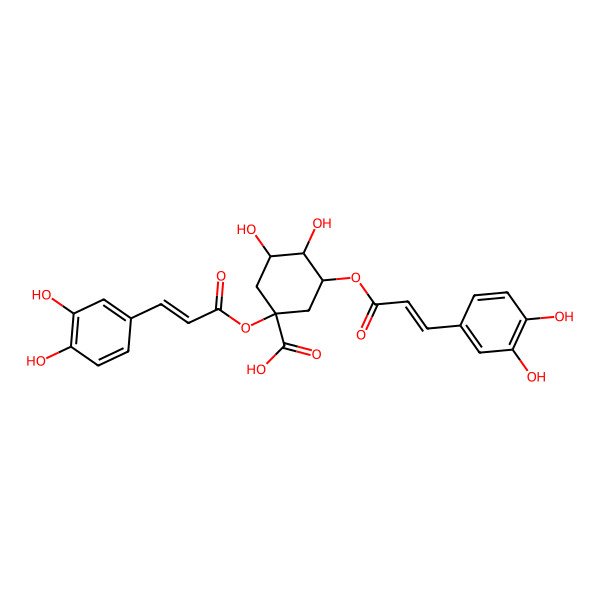
| Cynarine |
| Cinarine |
| 30964-13-7 |
| Cinarin |
| 1,5-Dicaffeoylquinic acid |
| 1,3-Dicaffeoylquinic acid |
| Cynarine [INN] |
| Plemocil |
| 1,3-Dicqa |
| Phemocil |
| 1,5-Dicaffeoylqunic acid |
| UNII-85D81U9JAV |
| Cynarine [MI] |
| 85D81U9JAV |
| (1R,3R,4S,5R)-1,3-Bis((3-(3,4-dihydroxyphenyl)acryloyl)oxy)-4,5-dihydroxycyclohexanecarboxylic acid |
| Cynarine [MART.] |
| 212891-05-9 |
| Cynarine [WHO-DD] |
| Quinic acid 1,5-dicaffeic ester |
| CHEBI:520 |
| Caffeic acid 1-carboxy-4,5-dihydroxy-1,3-cyclohexylene ester |
| 1-carboxy-4,5-dihydroxy-1,3-cyclohexylenebis-(3,4-dihydroxycinnamate) |
| Cynarine (MART.) |
| 1884-23-7 |
| 3,4-Dihydroxycinnamic acid 1-carboxy-4,5-dihydroxy-1,3-cyclohexylene ester |
| 3,4-Dihydroxycinnamic acid, 1-carboxy-4,5-dihydroxy-1,3-cyclohexylene ester |
| (1R,3R,4S,5R)-1,3-Bis((3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-4,5-dihydroxycyclohexanecarboxylic acid |
| (1R,3R,4S,5R)-1,3-bis[[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy]-4,5-dihydroxycyclohexane-1-carboxylic acid |
| Cyclohexanecarboxylic acid, 1,3-bis(((2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-4,5-dihydroxy-, (1R,3R,4S,5R)- |
| 1,5-di-O-Caffeoylquinic acid |
| DTXCID9029805 |
| Cynarin(e) |
| (1R,3R,4S,5R)-1,3-bis(((E)-3-(3,4-dihydroxyphenyl)acryloyl)oxy)-4,5-dihydroxycyclohexane-1-carboxylic acid |
| (1R,3R,4S,5R)-1,3-bis({[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy})-4,5-dihydroxycyclohexane-1-carboxylic acid |
| (1R-(1alpha,3alpha,4alpha,5beta))-1,3-Bis((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-4,5-dihydroxycyclohexanecarboxylic acid |
| Cyclohexanecarboxylic acid, 1,3-bis((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-4,5-dihydroxy- |
| CAS-30964-13-7 |
| 1,5-Dicaffeoyl quinic acid |
| DTXSID0050423 |
| cynarinum |
| Cynaricine |
| C25-H24-O12 |
| Cynarine [INN:WHO-DD] |
| Cynarin, analytical standard |
| SCHEMBL42528 |
| 1_5_dihydrocaffeoylquinic_acid |
| CHEMBL487258 |
| DTXSID301309674 |
| BCP29379 |
| Cyclohexanecarboxylic acid, 1,4-bis[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,5-dihydroxy- |
| HY-N0359 |
| Tox21_111176 |
| BDBM50369484 |
| s3301 |
| AKOS015912727 |
| Tox21_111176_1 |
| AC-6022 |
| CS-6280 |
| NCGC00274085-01 |
| AS-74959 |
| 1,3-Dicaffeoylquinic acid, >=98% (HPLC) |
| A820670 |
| J-003723 |
| Q-100389 |
| Q1763092 |
| Cynarine;1,3-Dicaffeoylquinic acid;1,5-DQA;1,5-Dicaffeoylquinic acid |
| (1R,3R,4S,5R)-1,3-Bis((3-(3,4-dihydroxyphenyl)acryloyl)oxy)-4,5-dihydroxycyclohexanecarboxylicacid |
| (1R,3R,4S,5R)-1,3-bis{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-4,5-dihydroxycyclohexane-1-carboxylic acid |
| (1R-(1.ALPHA.,3.ALPHA.,4.ALPHA.,5.BETA.))-1,3-BIS((3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPENYL)OXY)-4,5-DIHYDROXYCYCLOHEXANECARBOXYLIC ACID |
| 1,3-Dicaffeoylquinic acid (constituent of echinacea angustifolia root, echinacea pallida root, echinacea purpurea root and echinacea purpurea aerial parts) |
| 1,3beta-Bis[(E)-3-(3,4-dihydroxyphenyl)acryloyloxy]-4beta,5alpha-dihydroxycyclohexane-1alpha-carboxylic acid |
| Cyclohexanecarboxylic acid,1,3-bis[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-4,5-dihydroxy-,(1R,3R,4S,5R)- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|