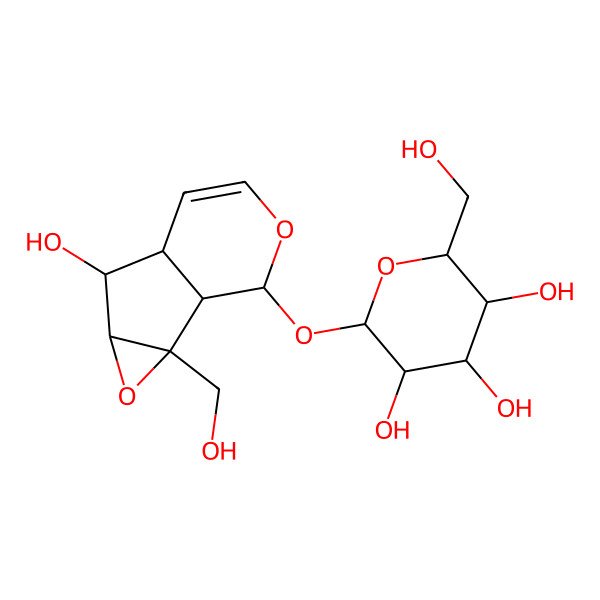
| Catalpinoside |
| 2415-24-9 |
| De(p-hydroxybenzoyl)catalposide |
| Catalposide, des-p-hydroxybenzoyl- |
| EINECS 219-324-0 |
| UNII-JCX5L7JIC2 |
| JCX5L7JIC2 |
| Catapol |
| CHEMBL513223 |
| CHEBI:69797 |
| DTXSID60178850 |
| beta-D-Glucopyranoside, 1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno(4,5)cyclopenta(1,2-c)pyran-2-yl, (1aS-(1aalpha,1bbeta,2beta,5abeta,6beta,6aalpha))- |
| (1AS-(1aalpha,1bbeta,2beta,5abeta,6beta,6aalpha))-1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno(4,5)cyclopenta(1,2-c)pyran-2-yl-beta-D-glucopyranoside |
| beta-D-Glucopyranoside, 1a,1b,2,5a,6,6a-hexahydro-6-hydroxy- 1a-(hydroxymethyl)oxireno(4,5)cyclopenta(1,2-c)pyran-2-yl, (1aS-(1a-alpha,1b-beta,2-beta,5a-beta,6-beta,6a-alpha))- |
| [1aS-(1aalpha,1bbeta,2beta,5abeta,6beta,6aalpha)]-1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-2-yl-beta-D-glucopyranoside |
| Digitalis purpurea L |
| SCHEMBL420515 |
| Catalpol, >=96% (HPLC) |
| DTXCID20101341 |
| HY-N0820 |
| BDBM50259972 |
| AKOS024264429 |
| AC-8052 |
| AM84820 |
| CCG-208299 |
| LMPR0102070007 |
| NCGC00163523-01 |
| AS-75213 |
| LS-71485 |
| SR-05000002315 |
| Q1050267 |
| SR-05000002315-2 |
| (1aS,1bS,2S,5aR,6S,6aS)-1a,1b,2,5a,6,6a-Hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-2-yl beta-D-glucopyranoside |
| (1S,5R,6R)-2-[(1S,1bS,2S,5aR,6S)-6-hydroxy-1a-((S)-hydroxymethyl)-1a,1b,2,5a,6,6a-hexahydro-1,3-dioxa-cyclopropa[a]inden-2-yloxy]-6-((S)-hydroxymethyl)-tetrahydro-pyran-3,4,5-triol |
| (2S,3R,4S,5S,6R)-2-(((1aS,1bS,2S,5aR,6S,6aS)-6-hydroxy-1a-(hydroxymethyl)-1a,1b,2,5a,6,6a-hexahydrooxireno[2',3':4,5]cyclopenta[1,2-c]pyran-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| (2S,3R,4S,5S,6R)-2-[[(1S,2S,4S,5S,6R,10S)-5-hydroxy-2-(hydroxymethyl)-3,9-dioxatricyclo[4.4.0.02,4]dec-7-en-10-yl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
| (2S,3R,4S,5S,6R)-2-{[(1S,2S,4S,5S,6R,10S)-5-HYDROXY-2-(HYDROXYMETHYL)-3,9-DIOXATRICYCLO[4.4.0.0(2),?]DEC-7-EN-10-YL]OXY}-6-(HYDROXYMETHYL)OXANE-3,4,5-TRIOL |
| .beta.-D-Glucopyranoside, (1aS,1bS,2S,5aR,6S,6aS)-1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-2-yl |
| beta-D-Glucopyranoside, 1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno(4,5)cyclopenta(1,2-c)pyran-2-yl, (1aS-(1a-alpha,1b-beta,2-beta,5a-beta,6-beta,6a-alpha))- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|