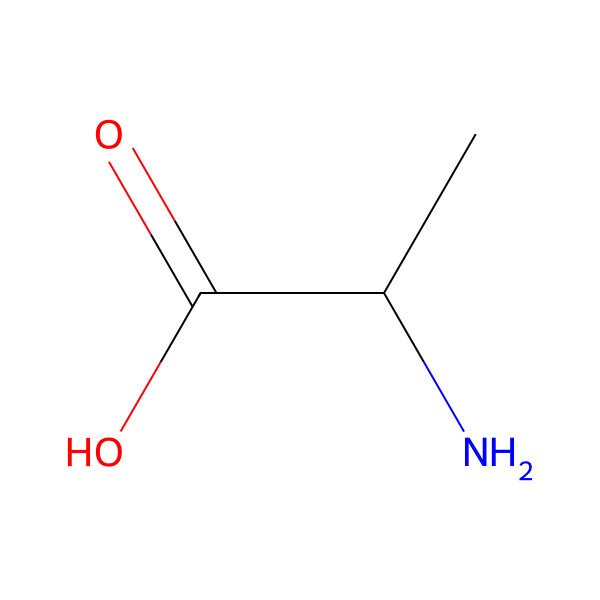
| alanine |
| 56-41-7 |
| (S)-Alanine |
| L-alpha-alanine |
| (S)-2-Aminopropanoic acid |
| (2S)-2-Aminopropanoic acid |
| L-2-Aminopropionic acid |
| H-Ala-OH |
| L-(+)-Alanine |
| L-2-Aminopropanoic acid |
| 2-Aminopropionic acid |
| alpha-Alanine |
| L-alpha-Aminopropionic acid |
| alpha-Aminopropionic acid |
| L-2-Aminopropionsaeure |
| (L)-Alanine |
| L-S-Aminopropionic acid |
| (S)-2-Aminopropionsaeure |
| 2-Aminopropanoic acid, L- |
| Alaninum [Latin] |
| alanina |
| (S)-(+)-Alanine |
| (S)-alpha-Aminopropionsaeure |
| Alaninum |
| Alanine (VAN) |
| ALANINE, L- |
| L-Alanin |
| Alanine [USAN:INN] |
| Alanina [DCIT,Spanish] |
| a-Alanine |
| Propanoic acid, 2-amino-, (S)- |
| L-a-Aminopropionic acid |
| L-a-Alanine |
| HSDB 1801 |
| (S)-2-Aminopropionic acid |
| 14C-L-Alanine |
| a-Aminopropionic acid |
| (C14)L-Alanine |
| NSC 206315 |
| UNII-OF5P57N2ZX |
| L-&alpha-alanine |
| EINECS 200-273-8 |
| OF5P57N2ZX |
| Alanine (USP) |
| L-Ala |
| ALA |
| 25191-17-7 |
| L-Alanine (9CI) |
| CHEBI:16977 |
| L-Alanine, labeled with carbon-14 |
| MFCD00064410 |
| CHEMBL279597 |
| L-Alanine, labeled with tritium |
| DTXSID20873899 |
| EC 200-273-8 |
| Propanoic acid, 2-amino-, (S) |
| Alaninum (Latin) |
| L-Alanine (JAN) |
| Alanine (L-Alanine) |
| LPG |
| [3H]alanine |
| ALANINE (II) |
| ALANINE [II] |
| L-ALANINE [JAN] |
| [14C]alanine |
| [3H]-alanine |
| [14C]-alanine |
| 26336-61-8 |
| l alanine |
| ALANINE (EP MONOGRAPH) |
| ALANINE [EP MONOGRAPH] |
| (2S)-2-aminopropanoate |
| ALANINE (USP MONOGRAPH) |
| ALANINE [USP MONOGRAPH] |
| .alpha.-Alanine |
| L-Isomer alanine |
| F4F207FF-8FF8-4789-99A1-147AE0A36673 |
| Alanine, L isomer |
| Alanine, L-isomer |
| 18875-37-1 |
| L-.alpha.-Alanine |
| NSC-206315 |
| SERINE IMPURITY A (EP IMPURITY) |
| SERINE IMPURITY A [EP IMPURITY] |
| VALINE IMPURITY A (EP IMPURITY) |
| VALINE IMPURITY A [EP IMPURITY] |
| 115967-49-2 |
| 130380-93-7 |
| 2-Ammoniopropanoate |
| Propanoic acid, 2-amino- |
| .alpha.-Aminopropionic acid |
| LYSINE ACETATE IMPURITY C (EP IMPURITY) |
| LYSINE ACETATE IMPURITY C [EP IMPURITY] |
| L-.alpha.-Aminopropionic acid |
| Ritalanine |
| Racemic alanine |
| L-alanina |
| a-Aminopropionate |
| L- alanine |
| 3h-l-alanine |
| L-Alanine Powder |
| L-a-Aminopropionate |
| alpha-Aminopropanoate |
| alpha-Aminopropionate |
| L-2-Aminopropanoate |
| L-2-Aminopropionate |
| L-Alanine,(S) |
| L-A-Aminopropionicacid |
| L-alpha-Aminopropionate |
| Tocris-0205 |
| 2-Ammoniopropanoic acid |
| ALA-OH |
| starbld0003382 |
| L-Alanine (JP17) |
| (S)-2-Aminopropanoate |
| ALANINE [VANDF] |
| ALANINE [HSDB] |
| ALANINE [INCI] |
| ALANINE [USAN] |
| ALANINE [INN] |
| alpha-Aminopropanoic acid |
| ALANINE [MI] |
| L-Ala-2 |
| L-ALANINE [FCC] |
| (S)-2-amino-Propanoate |
| ALANINE [WHO-DD] |
| L-Alanine, >=99% |
| bmse000028 |
| bmse000994 |
| D09PUL |
| L-CH3CH(NH2)COOH |
| L-ALANINE [USP-RS] |
| Alanine, L-(7CI,8CI) |
| GTPL720 |
| (S)-2-amino-Propanoic acid |
| (2S)-2-azanylpropanoic acid |
| Alanine, L- (7CI,8CI) |
| GTPL4542 |
| GTPL4543 |
| L-Alanine, >=98% (TLC) |
| L-Alanine, 99%, natural, FG |
| DTXCID001012092 |
| HY-N0229 |
| STR01663 |
| L-Alanine, >=99.0% (NT) |
| AC-014 |
| BDBM50000099 |
| s5631 |
| (S)-2-aminopropanoic acid;H-Ala-OH |
| AKOS010367904 |
| AKOS015840030 |
| CCG-266017 |
| CS-W020002 |
| DB00160 |
| L-Alanine, purum, >=98.0% (NT) |
| NCGC00024494-01 |
| 6898-94-8 |
| AC-24031 |
| BP-13281 |
| LS-15737 |
| L-Alanine, BioUltra, >=99.5% (NT) |
| L-Alanine, SAJ special grade, >=99.0% |
| A0179 |
| AM20100374 |
| EN300-52621 |
| A15652 |
| C00041 |
| D00012 |
| D84362 |
| L-Alanine, Cell Culture Reagent (H-L-Ala-OH) |
| A803529 |
| Q218642 |
| SR-01000597687 |
| J-015860 |
| Q-201274 |
| SR-01000597687-3 |
| (C23-H30-N2-O4.C15-H10-N2-O2)x- |
| L-Alanine, certified reference material, TraceCERT(R) |
| Alanine, European Pharmacopoeia (EP) Reference Standard |
| F0001-2354 |
| Z756424912 |
| L-Alanine, United States Pharmacopeia (USP) Reference Standard |
| L-Alanine, Pharmaceutical Secondary Standard; Certified Reference Material |
| L-Alanine, from non-animal source, meets EP, USP testing specifications, suitable for cell culture, >=98.5% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|