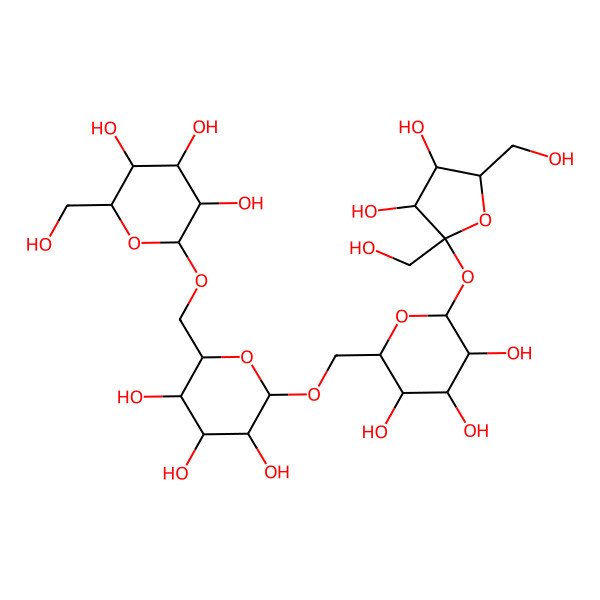
| D-Stachyose |
| 470-55-3 |
| Lupeose |
| CHEBI:17164 |
| stachyose hydrate |
| UNII-25VX64653N |
| EINECS 207-427-3 |
| 10094-58-3 |
| O-alpha-D-galactopyranosyl-(1->6)o-alpha-D-galactopyranosyl-(1->6)O-alpha-D-galactopyranosyl-beta-D-fructofuranoside |
| Manneotetrose |
| alpha-D-Galp-(1->6)-alpha-D-Galp-(1->6)-alpha-D-Glcp-(1->2)-beta-D-Fruf |
| alpha-D-Galp-(1->6)-alpha-D-Galp-(1->6)-alpha-D-Glcp-(1<->2)-beta-D-Fruf |
| beta-D-fructofuranosyl alpha-D-galactopyranosyl-(1->6)-alpha-D-galactopyranosyl-(1->6)-alpha-D-glucopyranoside |
| 54261-98-2 |
| Stachyose hydrate from Stachys tuberifera |
| DTXSID30889345 |
| (2R,3R,4S,5S,6R)-2-(((2R,3S,4S,5R,6R)-6-((2R,3R,4S,5R,6R)-6-((2S,3S,4R,5S)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl)oxy-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl)oxy-3,4,5-trihydroxy-oxan-2-yl)methoxy)-6-(hydroxymethyl)oxane-3,4,5-triol |
| (2R,3R,4S,5S,6R)-2-[[(2R,3S,4S,5R,6R)-6-[(2R,3R,4S,5R,6R)-6-[(2S,3S,4R,5S)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-3,4,5-trihydroxy-oxan-2-yl]methoxy]-6-(hydroxymethyl)oxane-3,4,5-triol |
| .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl O-.alpha.-D-galactopyranosyl-(1.fwdarw.6)-O-.alpha.-D-galactopyranosyl-(1.fwdarw.6)-, hydrate |
| bmse000240 |
| Epitope ID:150068 |
| C01613 |
| SCHEMBL32922 |
| CHEMBL1625803 |
| UQZIYBXSHAGNOE-XNSRJBNMSA-N |
| DTXCID601324144 |
| .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl O-.alpha.-D-galactopyranosyl-(1.fwdarw.6)-O-.alpha.-D-galactopyranosyl-(1.fwdarw.6)- |
| alpha-D-Glucopyranoside, beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1->6)-O-alpha-D-galactopyranosyl-(1->6)- |
| HY-N7910 |
| MFCD00006631 |
| AKOS016009597 |
| NCGC00246978-01 |
| CS-0138789 |
| S0397 |
| F77877 |
| Q283435 |
| GAL(alpha1->6)GAL(alpha1->6)GLC(alpha1->2beta)FRU |
| alpha-D-Gal-(1->6)-alpha-D-Gal-(1->6)-alpha-D-Glc-(1->2)-beta-D-Fru |
| alpha-D-Galp-(1->6)-alpha-D-Galp-(1->6)-alpha-D-Glcp-(12)-beta-D-Fruf |
| (2R,3R,4S,5S,6R)-2-(((2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yl)oxy)-6-((((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-((((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-3,4,5-triol |
| (2R,3R,4S,5S,6R)-2-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-({[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-({[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}methyl)oxane-3,4,5-triol |
| (2S,3R,4S,5R,6R)-2-{[(2R,3R,4S,5R,6S)-6-{[(2R,3S,4S,5R,6R)-6-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
| beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1->6)-O-alpha-D-galactopyranosyl-(1->6)-alpha-D-Glucopyranoside |
| beta-D-fructofuranosyl O-alpha-D-galactopyranosyl-(1-6)-O-alpha-D-galactopyranosyl-(1-6)-alpha-D-galactopyranoside |
| beta-D-Fructofuranosyl O-alpha-D-Galactopyranosyl-(1-6)-O-alpha-D-galactopyranosyl-(1-6)-alpha-D-glucopyranoside |
|
There are more than 10 synonyms. If you wish to see them all click here.
|