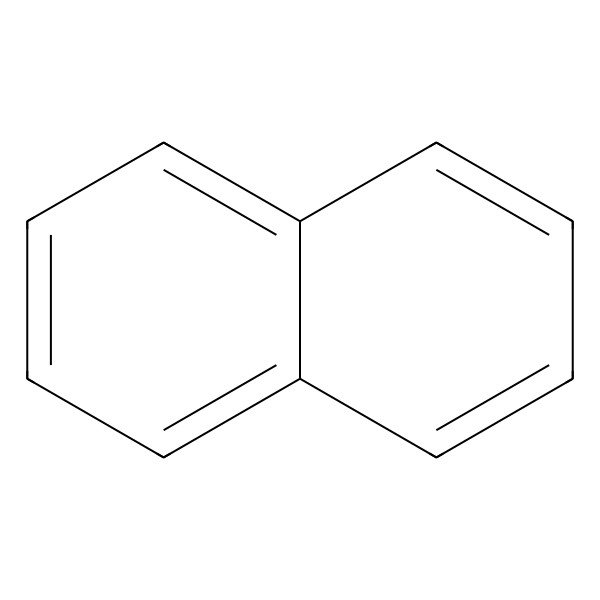
| 91-20-3 |
| Naphthalin |
| Tar camphor |
| White tar |
| Albocarbon |
| Naphthene |
| Camphor tar |
| Naphthaline |
| Moth balls |
| Moth flakes |
| naphtalene |
| Dezodorator |
| Naftalen |
| Mighty 150 |
| Naphthalinum |
| RCRA waste number U165 |
| Mighty RD1 |
| naftaleno |
| naftalina |
| naphtaline |
| naphthalen |
| napthalene |
| Naphtalinum |
| Mothballs |
| Caswell No. 587 |
| NCI-C52904 |
| Naphthalene, pure |
| Naftalen [Polish] |
| NSC 37565 |
| CCRIS 1838 |
| HSDB 184 |
| Naphthalene [BSI:ISO] |
| Naphtalene [ISO:French] |
| C10H8 |
| EINECS 202-049-5 |
| Naphthalene, crude |
| NSC-37565 |
| Naphthalene, molten |
| EPA Pesticide Chemical Code 055801 |
| UNII-2166IN72UN |
| Naphthalene (molten) |
| DTXSID8020913 |
| CHEBI:16482 |
| AI3-00278 |
| 2166IN72UN |
| UN1334 |
| UN2304 |
| RCRA waste no. U165 |
| Naphthalene, crude or refined |
| CHEMBL16293 |
| DTXCID00913 |
| NAPHTHALENE-1,4-D2 |
| EC 202-049-5 |
| NSC37565 |
| Naphthalene, 99% |
| Naphthalene-1,2,3,4-13C4 |
| MFCD00001742 |
| NCGC00090793-02 |
| NAPHTHALENE (IARC) |
| NAPHTHALENE [IARC] |
| NAPHTHALENE (MART.) |
| NAPHTHALENE [MART.] |
| Naphthalene, analytical standard |
| CAS-91-20-3 |
| 68412-25-9 |
| 287399-39-7 |
| NAPHTHALENE (1,2,3,4,5,6,7,8-D8) |
| 2-naphthalen |
| 1-Naphthalene |
| 2-Naphthalene |
| Na-Cemmix |
| Naphthalene,(S) |
| Naphthalene, 98% |
| NTM (CHRIS Code) |
| NAPHTHALENE [MI] |
| NAPHTHALENE [ISO] |
| NAPHTHALENE [HSDB] |
| NAPHTHALENE, REFINED |
| NAPHTHALINUM [HPUS] |
| NAPHTHALENE [USP-RS] |
| NAPHTHALENE [WHO-DD] |
| MLS001055498 |
| WLN: L66J |
| BIDD:ER0665 |
| HMS3039N15 |
| AMY22299 |
| Tox21_111023 |
| Tox21_202004 |
| Tox21_300008 |
| BDBM50159249 |
| NA1334 |
| NA2304 |
| Naphthalene, for synthesis, 98.5% |
| STL282720 |
| AKOS000119977 |
| USEPA/OPP Pesticide Code 055801 |
| LS-1917 |
| Naphthalene 100 microg/mL in Methanol |
| UN 1334 |
| UN 2304 |
| Naphthalene 10 microg/mL in Cyclohexane |
| NCGC00090793-01 |
| NCGC00090793-03 |
| NCGC00090793-04 |
| NCGC00090793-05 |
| NCGC00254058-01 |
| NCGC00259553-01 |
| BS-22320 |
| Naphthalene 10 microg/mL in Acetonitrile |
| SMR000677944 |
| Naphthalene 100 microg/mL in Acetonitrile |
| Naphthalene, SAJ first grade, >=98.0% |
| Bicyclo[4.4.0]deca-1,3,5,7,9-pentene |
| FT-0651884 |
| FT-0672611 |
| FT-0672612 |
| N0004 |
| N0885 |
| EN300-21626 |
| C00829 |
| D97670 |
| Naphthalene, suitable for scintillation, >=99% |
| L001166 |
| Naphthalene, molten [UN2304] [Flammable solid] |
| Naphthalene, molten [UN2304] [Flammable solid] |
| Q179724 |
| SR-01000854997 |
| Melting point standard 79-81C, analytical standard |
| SR-01000854997-2 |
| F0001-2217 |
| Naphthalene, certified reference material, TraceCERT(R) |
| Z104506008 |
| Naphthalene, crude or refined [UN1334] [Flammable solid] |
| Naphthalene, crude or refined [UN1334] [Flammable solid] |
| Naphthalene, United States Pharmacopeia (USP) Reference Standard |
| InChI=1/C10H8/c1-2-6-10-8-4-3-7-9(10)5-1/h1-8 |
| Naphthalene, Pharmaceutical Secondary Standard; Certified Reference Material |
| 25135-16-4 |
| 72931-45-4 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|