| vitamin B1 |
| thiamin |
| Aneurin |
| Antiberiberi factor |
| Thiadoxine |
| Betaxin |
| Biamine |
| Thiamine ion |
| 70-16-6 |
| Vitaneuron |
| Bequin |
| thiaminium |
| Bewon |
| thiamine(1+) |
| thiamine(1+) ion |
| Betabion |
| [3H]thiamine |
| [3H]-thiamine |
| [3H]vitamin B1 |
| Thiamine (Vit B1) |
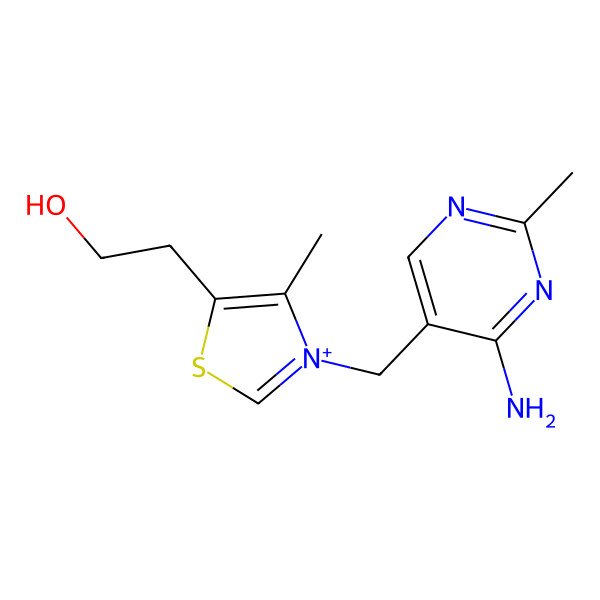
| 2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol |
| Vitamin B 1 |
| Thiamine hydrochloride [JAN] |
| 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium |
| 4ABT0J945J |
| Thiamine monohydrochloride |
| CHEBI:18385 |
| 3-(4-AMINO-2-METHYL-PYRIMIDIN-5-YLMETHYL)-5-(2-HYDROXY-ETHYL)-4-METHYL-THIAZOL-3-IUM |
| Chloride-hydrochloride salt of thiamine |
| THD |
| VIB |
| Vitamin B1 hydrochloride (VAN) |
| Thiamine, chloride, hydrochloride |
| Vitamin B1 (TN) |
| vitamin b1(thiamine) |
| UNII-4ABT0J945J |
| 3-((4-Amino-2-methylpyrimidin-5-yl)methyl)-5-(2-hydroxyethyl)-4-methylthiazol-3-ium |
| 3[(4-Amino-2-methyl-5-pyrimidinyl)-methyl]-5-(2-hydroxyethyl)-4-methylthiazolium chloride |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl- |
| NSC36226 |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl-, chloride, monohydrochloride |
| THIAMIN, VITAMIN B1 |
| 1sbr |
| 3rlb |
| ThOH |
| 3-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m- ethylthiazolium chloride, monohydrochloride |
| 3-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m-ethylthiazolium chloride, monohydrochloride |
| Prestwick0_000631 |
| Prestwick1_000631 |
| Prestwick2_000631 |
| Prestwick3_000631 |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m- ethyl, chloride, monohydrochloride |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m-ethyl, chloride, monohydrochloride |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl-chloride (1:1) |
| bmse000274 |
| D06PQT |
| D07TYL |
| TimTec1_000613 |
| CHEMBL1547 |
| THIAMIN; VITAMIN B1 |
| SCHEMBL10075 |
| BSPBio_000622 |
| SPBio_002841 |
| BPBio1_000686 |
| C12H17N4OS |
| GTPL4628 |
| GTPL4629 |
| SCHEMBL22129283 |
| DTXSID50220251 |
| JZRWCGZRTZMZEH-UHFFFAOYSA-N |
| BDBM50373877 |
| STL301841 |
| AKOS000668650 |
| DB00152 |
| LS-2340 |
| 2-[3-[(4-amino-2-methyl-pyrimidin-5-yl)methyl]-4-methyl-thiazol-3-ium-5-yl]ethanol |
| SMP1_000084 |
| NCGC00017013-06 |
| NCGC00188957-01 |
| NCGC00188957-02 |
| LT00233141 |
| C00378 |
| Q83187 |
| 3-(2-Methyl-4-aminopyrimidine-5-ylmethyl)-4-methylthiazolium-5-ethanol |
| [5-[[5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium-3-yl]methyl]-2-methylpyrimidin-4-yl]azanium dichloride |
| 2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol chloride |
|
There are more than 10 synonyms. If you wish to see them all click here.
|