| 502-65-8 |
| all-trans-Lycopene |
| Psi,psi-carotene |
| trans-Lycopene |
| Lycopene 7 |
| lycored |
| Redivivo |
| Mexoryl SAQ |
| Tomat-O-Red |
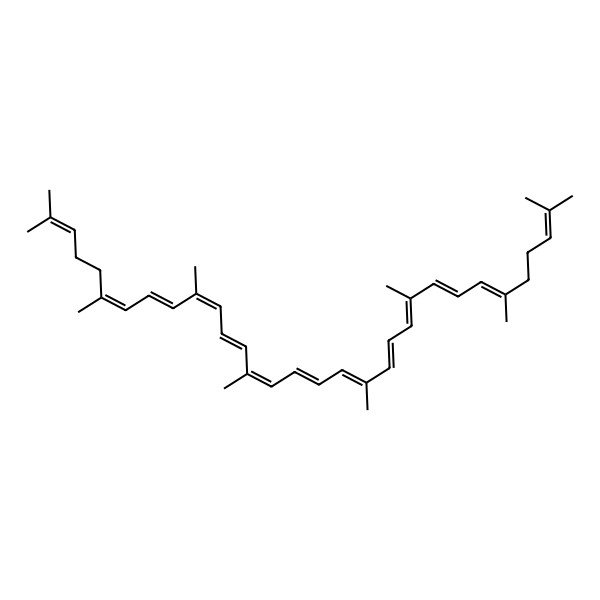
| (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene |
| (all-trans)-lycopene |
| Lycopene, all-trans- |
| TOMATO LYCOPENE |
| Aec lycopene |
| (all-e)-lycopene |
| Lycopene (VAN) |
| CI 75125 |
| Solanorubin |
| Ateronon |
| CCRIS 7925 |
| NSC 407322 |
| LYC-O-MATO |
| UNII-SB0N2N0WV6 |
| Blakeslea trispora |
| C.I. 75125 |
| EINECS 207-949-1 |
| SB0N2N0WV6 |
| INS-160D(III) |
| INS NO.160D(III) |
| Lycopene from blakeslea trispora |
| DTXSID2046593 |
| FEMA NO. 4110 |
| CHEBI:15948 |
| E-160D(III) |
| .psi.,.psi.-Carotene |
| C40H56 |
| NSC-407322 |
| DTXCID0026593 |
| NSC407322 |
| (all-E)-2,6,10,14,19,23,27,31-Octamethyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene |
| NCGC00166291-01 |
| LYCOPENE (MART.) |
| LYCOPENE [MART.] |
| cis-Lycopene |
| LYCOPENE PREPARATION (USP-RS) |
| LYCOPENE PREPARATION [USP-RS] |
| MFCD00017350 |
| All trans Lycopene |
| psi, psi-Carotene |
| LyocpenePowder |
| psi-psi-carotene |
| y,y-Carotene |
| Lyco Vit |
| LYC |
| Lycopene preparation |
| Lycopene all-trans- |
| Psi, psi- carotene |
| LYCOPENE [INCI] |
| LYCOPENE [MI] |
| LYCOPENE [VANDF] |
| LYCOPENE [WHO-DD] |
| BSPBio_003389 |
| Lycopene, analytical standard |
| E160d |
| TOMATO LYCOPENE [FHFI] |
| CHEMBL501174 |
| Lycopene, >=90%, from tomato |
| CI 75125 [INCI] |
| HY-N0287 |
| Tox21_112395 |
| LMPR01070257 |
| s3943 |
| AKOS015961276 |
| CS-6378 |
| DB11231 |
| FD10111 |
| NCGC00166291-02 |
| NCGC00166291-03 |
| NCGC00166291-04 |
| 2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene |
| AC-13571 |
| AC-33932 |
| CAS-502-65-8 |
| LS-15428 |
| Lycopene, >=98% (HPLC), from tomato |
| LS-175138 |
| L0257 |
| LYCOPENE FROM BLAKESLEA TRISPORA [FCC] |
| C05432 |
| Q208130 |
| Q-100561 |
| Lycopene, United States Pharmacopeia (USP) Reference Standard |
| Lycopene, Pharmaceutical Secondary Standard; Certified Reference Material |
| (ALL-E)-LYCOPENE (CONSTITUENT OF LYCOPENE AND TOMATO EXTRACT CONTAINING LYCOPENE) |
| (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-Octamethyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene |
| 2,6,8,10,12,14,16,18,20,22,24,26,30-Dotriacontatridecaene, 2,6,10,14,19,23,27,31-octamethyl-, (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)- |
| 2,6,8,10,12,14,16,18,20,22,24,26,30-Dotriacontatridecaene, 2,6,10,14,19,23,27,31-Octamethyl-, (all-E)- |
| LYC, LYCOPENE, Lycopene 7, Lycopene all-trans-, Lycopene, all-trans-, Lycopene, all-trans- (8CI), Lycopene (VAN), Natural yellow 27, NCGC00166291-01, NCGC00166291-02, NSC407322, NSC 407322 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|