| 520-27-4 |
| Barosmin |
| Diosimin |
| Venosmine |
| Diosmil |
| Flebosten |
| Diosmine |
| Daflon |
| Tovene |
| Ven-Detrex |
| Diovenor |
| Buchu Resin |
| Litosmil |
| Veno-V |
| flebosmil |
| Diosminum |
| Rioven |
| Diosmetin 7-O-rutinoside |
| Diosven |
| Flebaven |
| Flebavena |
| Hemerven |
| Insuven |
| Varinon |
| Dioven |
| Diosmetin-7-O-rutinoside |
| Diosmetin 7-rutinoside |
| UNII-7QM776WJ5N |
| Diosmin [INN] |
| 7QM776WJ5N |
| CCRIS 7915 |
| CHEBI:4631 |
| DTXSID4045892 |
| Diosmin [INN:BAN] |
| Diosmin [INN-Spanish] |
| Diosmine [INN-French] |
| Diosminum [INN-Latin] |
| EINECS 208-289-7 |
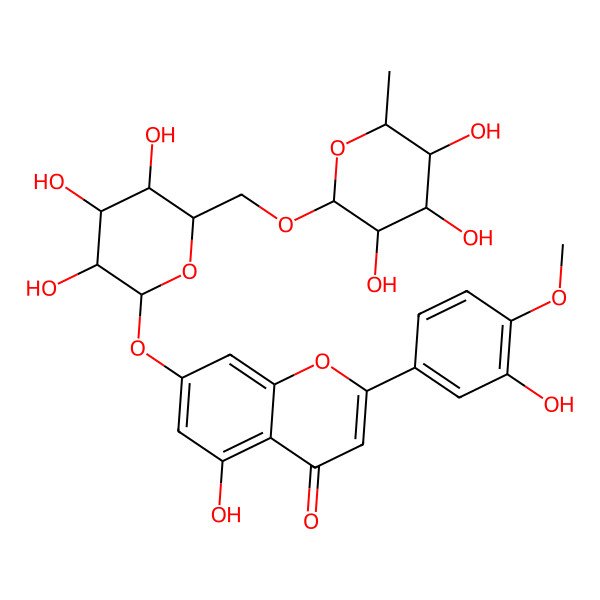
| 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one |
| NSC-758417 |
| 3',5,7-trihydroxy-4'-methoxyflavone-7-rutinoside |
| SE 4601 |
| DTXCID2025892 |
| 3',5,7-Trihydroxy-4'-methoxyflavone 7-rutinoside |
| Diosmin (INN) |
| NSC 758417 |
| 3',5,7-trihydroxy-4'-methoxyflavone 7-rhamnoglucoside |
| 3',5,7-Trihydroxy-4'-methoxyflavone-7-(6-O-(-deoxy-alpha-L-mannopyraonsyl)-beta-D-glucopyranoside |
| 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one |
| 3',5-Dihydroxy-4'-methoxy-4-oxo-4H-chromen-7-ylrutosid |
| DIOSMIN (MART.) |
| DIOSMIN [MART.] |
| DIOSMIN (USP-RS) |
| DIOSMIN [USP-RS] |
| Diosmin, analytical standard |
| 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)- |
| 5-hydroxy-2-(3-hydroxy-4-methoxy-phenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl]oxymethyl]tetrahydropyran-2-yl]oxy-chromen-4-one |
| 5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((6-O-alpha-L-rhamnopyranosyl-beta-D-glycopyranosyl)oxy)-4-chromenon |
| DIOSMIN (EP MONOGRAPH) |
| DIOSMIN [EP MONOGRAPH] |
| 3',5-Dihydroxy-4'-methoxy-4-oxo-4H-chromen-7-ylrutosid [IUPAC] |
| Resin, Buchu |
| 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-4H-1-benzopyran-7-yl 6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside |
| 7-((6-O-(6-Deoxy-ga-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one |
| CAS-520-27-4 |
| SR-01000799147 |
| C28H32O15 |
| Diosmina |
| 3',5-Dihydroxy-4'-methoxy-4-oxo-4H-chromen-7-ylrutosid (IUPAC) |
| NCGC00095022-01 |
| 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-.alpha.-L-mannopyranosyl)-.beta.-D-glucopyranosyl)oxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)- |
| 4H-1-Benzopyran-4-one, 7-[[6-O-(6-deoxy-.alpha.-L-mannopyranosyl)-.beta.-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)- |
| Daflon (TN) |
| MFCD00009772 |
| Diosmin (8) |
| DIOSMINE [INCI] |
| DIOSMIN [DSC] |
| DIOSMIN [MI] |
| DIOSMIN [WHO-DD] |
| MLS001304032 |
| SCHEMBL120870 |
| CHEMBL231884 |
| C05CA03 |
| BDBM153267 |
| GLXC-13353 |
| HMS2233P16 |
| HMS3713L08 |
| Tox21_111392 |
| s2292 |
| AKOS015969767 |
| Tox21_111392_1 |
| BCP9000612 |
| CCG-208570 |
| DB08995 |
| SMP1_000183 |
| NCGC00344564-01 |
| 4H-1-Benzopyran-4-one,7-[[6-O-(6-deoxy-a-L-mannopyranosyl)-b-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)- |
| AS-13224 |
| BP-12422 |
| SMR000718616 |
| BCP0726000067 |
| LS-193056 |
| C10039 |
| D07858 |
| EN300-18388399 |
| 3',5,7-trihydroxy-4'-methoxy flavone-7-rutinoside |
| Q2607865 |
| SR-01000799147-4 |
| SR-01000799147-5 |
| SR-01000799147-6 |
| Diosmin, European Pharmacopoeia (EP) Reference Standard |
| Diosmin, United States Pharmacopeia (USP) Reference Standard |
| Diosmin for system suitability, European Pharmacopoeia (EP) Reference Standard |
| 4h-1-benzopiran-4-ona, 7-[[6-o-(6-deoxi-?-l-mannopiranosil)-?-d-glucopiranosil]oxi]-5-hidroxi-2-(3-hidroxi-4-metoxifenil)- |
| 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one |
| 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(O6-alpha-L-rhamnopyranosyl-beta-D-glucopyranosyloxy)chromen-4-one |
| 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one |
| 5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-7-{[(2S,3R,4S,5S,6R)-3,4,5-TRIHYDROXY-6-({[(2R,3R,4R,5R,6S)-3,4,5-TRIHYDROXY-6-METHYLOXAN-2-YL]OXY}METHYL)OXAN-2-YL]OXY}-4H-CHROMEN-4-ONE |
| 7-((6-O-(6-DEOXY-GA-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE |
|
There are more than 10 synonyms. If you wish to see them all click here.
|