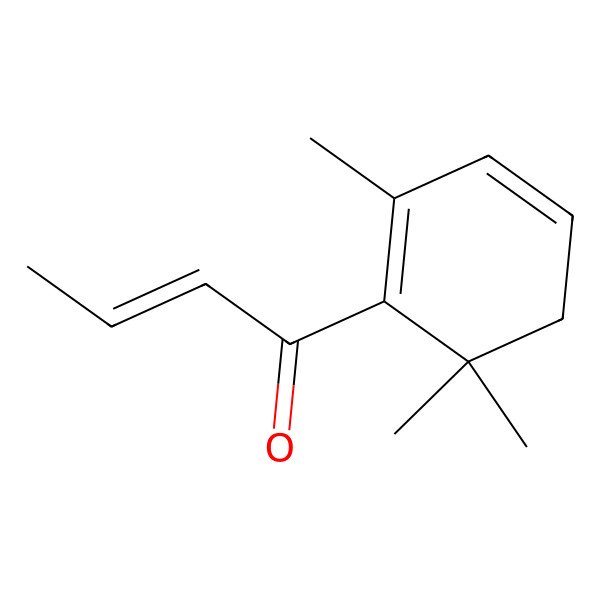
| beta-Damascenone |
| 23696-85-7 |
| 23726-93-4 |
| trans-beta-Damascenone |
| trans-damascenone |
| .beta.-Damascenone |
| 1-(2,6,6-Trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one |
| 2-BUTEN-1-ONE, 1-(2,6,6-TRIMETHYL-1,3-CYCLOHEXADIEN-1-YL)- |
| (E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one |
| FEMA No. 3420 |
| (E)-beta-damascenone |
| Damascenone (natural) |
| (E)-.beta.-Damascenone |
| UNII-U66V25TBO0 |
| U66V25TBO0 |
| (2E)-1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one |
| (E)-1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one |
| 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-, (2E)- |
| EINECS 245-833-2 |
| EINECS 245-844-2 |
| 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-2-en-4-one |
| 1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one |
| 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-, (E)- |
| 2,6,6-Trimethyl-1-trans-crotonoyl-1,3-cyclohexadiene |
| 1-[2,6,6-Trimethyl-1,3-cyclohexadien-1-yl]-2E-buten-1-one |
| (2E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one |
| 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-, (E) |
| DTXSID6041397 |
| fermentone |
| Damascenone, trans- |
| damascenone (e-beta) |
| beta-(E)-damascenone |
| 2,6,6-Trimethyl-1-crotonyl-1,3-cyclohexadiene |
| ?-Damascenone, 90% |
| trans-.beta.-Damascenone |
| .beta.-(E)-Damascenone |
| DAMASCENONE,.BETA.- |
| SCHEMBL80947 |
| DISCONTINUED, UNSTABLE" |
| trans-2,6,6-Trimethyl-1-crotonylcyclohexa-1,3-diene |
| Damascenone, analytical standard |
| TRANS-.BETA.-DAMASCENON |
| CHEMBL3733030 |
| CHEBI:67251 |
| .BETA.-DAMASCENONE [MI] |
| DTXCID80196511 |
| DTXSID90885242 |
| .BETA.-DAMASCENON, TRANS- |
| 1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one; 1-(2,6,6-Trimethyl-1,3-cyclohexadienyl)-2-buten-1-one; 1-Crotonoyl-2,6,6-trimethyl-1,3-cyclohexadiene; 2,6,6-Trimethyl-1-(2-butenoyl)-1,3-cyclohexadiene |
| HY-N2543 |
| Tox21_304001 |
| LMPR01070304 |
| MFCD00101024 |
| s6431 |
| AKOS015914961 |
| NCGC00357220-01 |
| AC-34815 |
| BS-16271 |
| CAS-23696-85-7 |
| CS-0022815 |
| 1-crotonoyl-2,6,6-trimethylcyclohexa-1,3-diene |
| 2,6,6-trimethyl-1-crotonoyl-1,3-cyclohexadiene |
| A816852 |
| Q416126 |
| (E)-beta-Damascenone 1000 microg/mL in Acetonitrile |
| Damascenone, certified reference material, TraceCERT(R) |
| 1-(2,6,6-trimethylcyclohexa-1,3-dienyl)-2-buten-1-one |
| 4-(2,6,6-Trimethyl cyclohexa-1,3-dienyl)but-2-en-4-one |
| (E)-1-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-1-one |
| 2-buten-1-ona, 1-(2,6,6-trimetil-1,3-ciclohexadien-1-il)- |
| (e)-1-(2,6,6-trimethyl-1-cyclohexa-1,3-dienyl)but-2-en-1-one |
| Damascenone, natural, 1.1-1.3 wt. % (190 proof ethanol), FG |
| (2 E)-1-(2,6,6-trimethylcyclohexane-1,3-dien-1-yl)but-2-en-1-one |
| 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one, trans- |
| 2-buten-1-ona, 1-(2,6,6-trimetil-1,3-ciclohexadien-1-il)-, (2e)- |
| 4-(2,6,6-TRIMETHYL CYCLOHEXA-1,3-DIENYL) BUT-2-EN-4-ONE [FHFI] |
| 1 - (2,6,6 - trimethyl - 1,3 - cyclohexadien - 1 - yl) - 2 - buten - 1 - one |
|
There are more than 10 synonyms. If you wish to see them all click here.
|