| 458-35-5 |
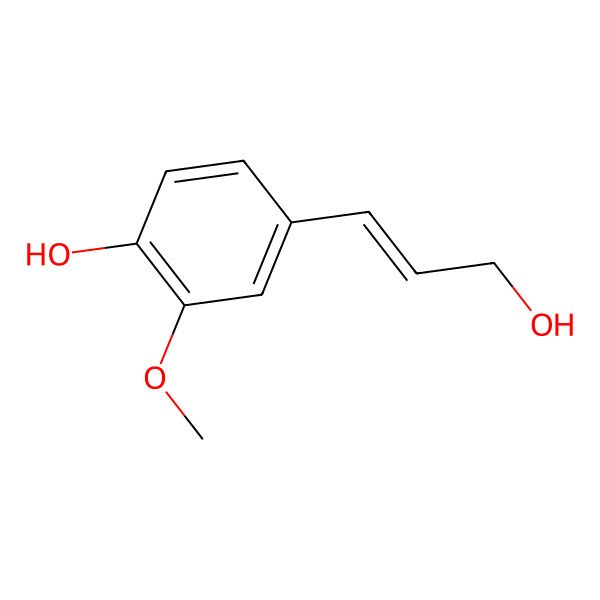
| Coniferol |
| 4-(3-hydroxyprop-1-en-1-yl)-2-methoxyphenol |
| 32811-40-8 |
| (E)-Coniferyl alcohol |
| trans-Coniferyl alcohol |
| gamma-Hydroxyisoeugenol |
| Coniferyl alcohol, E- |
| 4-Hydroxy-3-methoxycinnamic alcohol |
| 3-(4-Hydroxy-3-methoxyphenyl)-2-propen-1-ol |
| 4-(3-Hydroxy-1-propenyl)-2-methoxyphenol |
| 4-[(1E)-3-hydroxyprop-1-en-1-yl]-2-methoxyphenol |
| Coniferyl alcohol [MI] |
| 4-Hydroxy-3-methoxycinnamyl alcohol |
| UNII-E7SM92591P |
| trans-coniferol |
| CHEBI:17745 |
| E7SM92591P |
| (e)-coniferol |
| Coniferylic alcohol |
| E-Coniferyl alcohol |
| .gamma.-Hydroxyisoeugenol |
| EINECS 207-277-9 |
| 4-(3-Hydroxy-1-propen-1-yl)-2-methoxyphenol |
| 4-[(E)-3-hydroxyprop-1-enyl]-2-methoxyphenol |
| 4-[(1E)-3-Hydroxy-1-propenyl]-2-methoxyphenol |
| 4-Hydroxy-3-methoxycinnamylic alcohol |
| p-Hydroxy-m-methoxycinnamyl alcohol |
| 2-Propen-1-ol, 3-(4-hydroxy-3-methoxyphenyl)- |
| 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol |
| Phenol, 4-(3-hydroxy-1-propenyl)-2-methoxy- |
| Phenol, 4-(3-hydroxy-1-propenyl)-2-methoxy-, (E)- |
| 4-(3-hydroxyprop-1-enyl)-2-methoxyphenol |
| AI3-36149 |
| 3-(4-Hydroxy-3-methoxyphenyl)allyl alcohol |
| 2-Propen-1-ol, 3-(4-hydroxy-3-methoxyphenyl), (E)- |
| 4-((1E)-3-HYDROXY-1-PROPEN-1-YL)-2-METHOXYPHENOL |
| PHENOL, 4-((1E)-3-HYDROXY-1-PROPENYL)-2-METHOXY- |
| 2-PROPEN-1-OL, 3-(4-HYDROXY-3-METHOXYPHENYL)-, (E)- |
| 4-hydroxy-3-methoxy cinnamyl alcohol |
| 4-[3-Hydroxy-1-propenyl]-2-methoxyphenol |
| MFCD00002922 |
| 4-((1E)-3-hydroxyprop-1-en-1-yl)-2-methoxyphenol |
| DTXSID9060029 |
| Coniferyl?alcohol |
| CONIFERYL-ALCOHOL |
| epsilon-coniferyl alcohol |
| Coniferyl alcohol, 98% |
| Phenol, 4-(3-hydroxy-1-propen-1-yl)-2-methoxy- |
| bmse000602 |
| bmse010248 |
| bmse010285 |
| Epitope ID:116871 |
| (E)-4-(3-hydroxyprop-1-enyl)-2-methoxyphenol |
| SCHEMBL177683 |
| CHEMBL501870 |
| DTXCID7040447 |
| DTXSID50186489 |
| 4e70 |
| JMFRWRFFLBVWSI-UHFFFAOYSA-N |
| HY-N4283 |
| AKOS005258118 |
| PS-4366 |
| AC-34753 |
| 4-(3-hydroxy-1-propenyl)-2-methoxy-Phenol |
| CS-0032634 |
| S6429 |
| C00590 |
| D93191 |
| EN300-1857771 |
| EN300-6472482 |
| (E)-4-(3 -hydroxyprop-1-enyl)-2-methoxyphenol |
| 4-[(E)-3-hydroxyprop-1-enyl]-2-methoxy-phenol |
| A826901 |
| Q418993 |
| (E) 3-(4-hydroxy-3-methoxyphenyl)-2-Propen-1-ol |
| (E)-4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenol |
| F8420572-6986-4EF3-917B-3298FD384F2C |
|
There are more than 10 synonyms. If you wish to see them all click here.
|