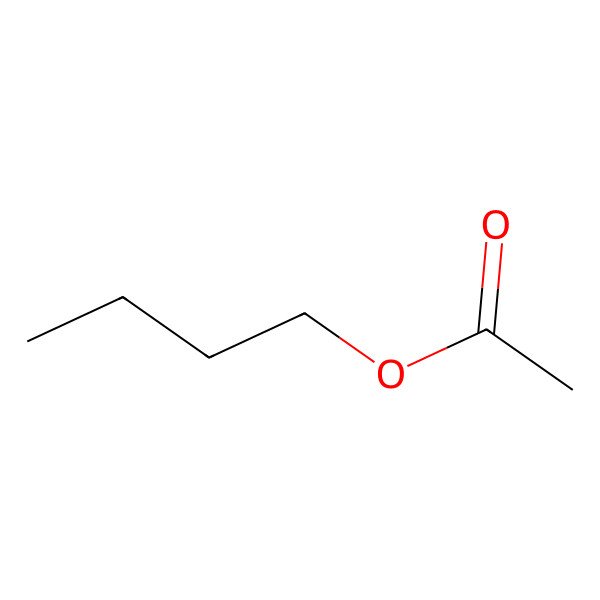
| N-BUTYL ACETATE |
| 123-86-4 |
| Acetic acid, butyl ester |
| Butyl ethanoate |
| 1-Butyl acetate |
| Acetic Acid Butyl Ester |
| n-Butylacetate |
| n-Butyl ethanoate |
| Butylacetat |
| Acetic acid n-butyl ester |
| Acetate de butyle |
| Butylacetaten |
| 1-acetoxybutane |
| Octan n-butylu |
| Butyl acetate, n- |
| Butylacetat [German] |
| Butyle (acetate de) |
| Butylacetaten [Dutch] |
| Butylester kyseliny octove |
| Octan n-butylu [Polish] |
| n-Butyl acetate (natural) |
| Butyl ester of acetic acid |
| NSC 9298 |
| Acetate de butyle [French] |
| Butile(acetati di) |
| 1-Butanol, acetate |
| Butyle (acetate de) [French] |
| CCRIS 2287 |
| HSDB 152 |
| Butile (acetati di) |
| Butile (acetati di) [Italian] |
| Butylester kyseliny octove [Czech] |
| EINECS 204-658-1 |
| MFCD00009445 |
| UN1123 |
| BRN 1741921 |
| CH3COO(CH2)3CH3 |
| n-Butyl acetate, HPLC Grade |
| ACETIC ACID,BUTYL ESTER |
| AI3-00406 |
| DTXSID3021982 |
| CHEBI:31328 |
| UNII-464P5N1905 |
| NSC-9298 |
| 1-Butylacetate |
| 464P5N1905 |
| EC 204-658-1 |
| 4-02-00-00143 (Beilstein Handbook Reference) |
| n-Butylacetat |
| n-butyl-acetate |
| nBuOAc |
| acetic acid butyl |
| AcOBu |
| BuOAc |
| n- butyl acetate |
| n-BuOAc |
| Butyle(acetate de) |
| n - butyl acetate |
| Essigsaeurebutylester |
| normal-butyl acetate |
| Nat. Butyl Acetate |
| ACETATE, BUTYL |
| Actate de butyle normal |
| BCN (CHRIS Code) |
| Essigsaeure-n-butylester |
| Butyl ester, acetic acid |
| Acetic acid, n-butyl ester |
| BUTYL ACETATE [FCC] |
| SCHEMBL14969 |
| BUTYL ACETATE [FHFI] |
| BUTYL ACETATE [INCI] |
| n-Butyl ester of acetic acid |
| WLN: 4OV1 |
| N-BUTYL ACETATE [MI] |
| n-Butyl acetate, ACS reagent |
| BUTYL ACETATE [MART.] |
| BUTYL ESTER ACETIC ACID |
| Butyl acetate, AR, 99.5% |
| Butyl acetate, LR, >=98% |
| CHEMBL284391 |
| DTXCID101982 |
| BUTYL ACETATE [USP-RS] |
| N-BUTYL ACETATE [HSDB] |
| FEMA NO. 2174 |
| Butyl Acetate (Fragrance Grade) |
| Butyl Acetate Reagent ACS Grade |
| Butyl Acetate (Industrial Grade) |
| n-Butyl acetate Biochemical grade |
| NSC9298 |
| Butyl acetate, ampule of 100 mg |
| Butyl acetate, analytical standard |
| Butyl acetate, anhydrous, >=99% |
| AMY11075 |
| Butyl acetate, for HPLC, 99.7% |
| n-Butyl acetate, analytical standard |
| Tox21_201052 |
| LS-684 |
| n-Butyl acetate, Semiconductor Grade |
| NA1123 |
| STL282735 |
| Butyl acetate, >=99%, FCC, FG |
| AKOS000120198 |
| Butyl acetate, natural, >=98%, FG |
| Butyl acetate, ACS reagent, >=99.5% |
| Butyl acetate, ReagentPlus(R), 99.5% |
| NCGC00091573-01 |
| NCGC00091573-02 |
| NCGC00258605-01 |
| CAS-123-86-4 |
| Butyl acetate, puriss. p.a., ACS reagent |
| A0024 |
| A0228 |
| Butyl acetate, SAJ first grade, >=98.0% |
| FT-0621752 |
| Butyl acetate, JIS special grade, >=99.0% |
| EN300-21265 |
| n-Butylacetate 100 microg/mL in Acetonitrile |
| n-Butyl acetate [UN1123] [Flammable liquid] |
| n-Butyl acetate [UN1123] [Flammable liquid] |
| A805161 |
| Q411073 |
| J-004991 |
| J-519958 |
| Q-200771 |
| TRIBUTYL ACETYLCITRATE IMPURITY E [EP IMPURITY] |
| F0001-0371 |
| Butyl acetate, >=99.5%, suitable for atomic absorption spectrometry |
| Butyl acetate, United States Pharmacopeia (USP) Reference Standard |
| InChI=1/C6H12O2/c1-3-4-5-8-6(2)7/h3-5H2,1-2H |
| Butyl acetate, Pharmaceutical Secondary Standard; Certified Reference Material |
| 8JZ |
|
There are more than 10 synonyms. If you wish to see them all click here.
|