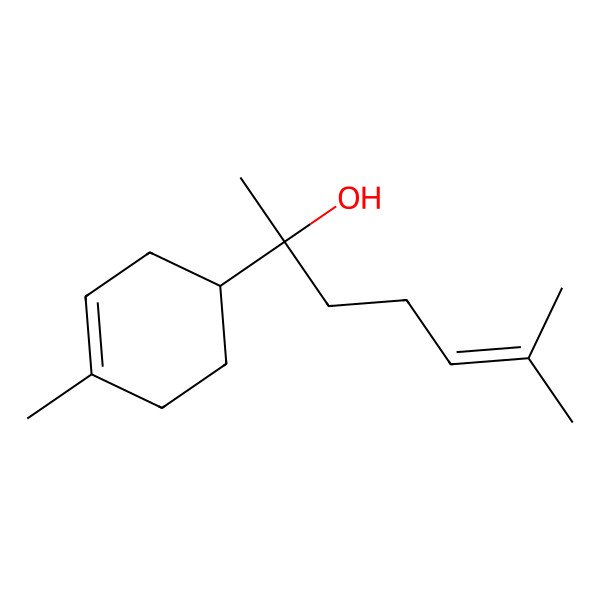
| Bisabolol |
| (+)-alpha-Bisabolol |
| 515-69-5 |
| 23178-88-3 |
| D-alpha-Bisabolol |
| (2R)-6-methyl-2-[(1R)-4-methylcyclohex-3-en-1-yl]hept-5-en-2-ol |
| (+)-(1'R,2R)-alpha-Bisabolol |
| alpha-Bisabolol, (+)- |
| UNII-36HQN158VC |
| Dragosantol |
| Camilol |
| a-Bisabolol |
| 36HQN158VC |
| CHEMBL1171719 |
| DTXSID5045964 |
| Hydagen B |
| UNII-105S6I733Z |
| alpha-Bisabolol (+)-form [MI] |
| MFCD03846910 |
| 105S6I733Z |
| (2R)-6-Methyl-2-(4-methyl-3-cyclohexenyl)-5-heptene-2-ol |
| (R)-6-Methyl-2-((R)-4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol |
| 5-HEPTEN-2-OL, 6-METHYL-2-(4-METHYL-3-CYCLOHEXEN-1-YL)- |
| alpha,4-Dimethyl-alpha-(4-methyl-3-pentenyl)-3-cyclohexene-1-methanol |
| 3-Cyclohexene-1-methanol, .alpha.,4-dimethyl-.alpha.-(4-methyl-3-pentenyl)-, (R*,R*)- |
| DTXCID3025964 |
| EINECS 208-205-9 |
| EINECS 246-973-7 |
| rel-(R)-6-Methyl-2-((R)-4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol |
| dl-.alpha.-Bisabolol |
| 3-Cyclohexene-1-methanol, alpha,4-dimethyl-alpha-(4-methyl-3-penten-1-yl)-, (alphaR,1R)- |
| 25428-43-7 |
| 3-Cyclohexene-1-methanol, alpha,4-dimethyl-alpha-(4-methyl-3-penten-1-yl)-, (alphaR,1R)-rel- |
| BISABOLOL, ALPHA |
| CAS-515-69-5 |
| BISABOLOL, .ALPHA. |
| RACEMIC ALFA-BISABOLOL |
| BISABOLA-1,12-DIEN-8-OL |
| (R*,R*)-alpha,4-Dimethyl-alpha-(4-methyl-3-pentenyl)cyclohex-3-ene-1-methanol |
| NCGC00095252-01 |
| (+)-a-Bisabolol |
| (+)-.alpha.-Bisabolol |
| BISABOLOL [WHO-DD] |
| D-.ALPHA.-BISABOLOL |
| SCHEMBL24988 |
| (AlphaR,1R)-Alpha-Bisabolol |
| (+)-6R,7R-alpha-Bisabolol |
| .alpha.,4-Dimethyl-.alpha.-(4-methyl-3-pentenyl)-3-cyclohexene-1-methanol |
| .ALPHA.-BISABOLOL [MI] |
| 3-Cyclohexene-1-methanol, ?,4-dimethyl-?-(4-methyl-3-pentenyl)-, (R,R)- |
| 3-Cyclohexene-1-methanol, alpha,4-dimethyl-alpha-(4-methyl-3-pentenyl)- |
| BISABOLOL, (+/-)- |
| (alpha R,1R)-rel-alpha,4-Dimethyl-alpha-(4-methyl-3-penten-1-yl)-3-cyclohexene-1-methanol |
| (R*, R*)- a, 4- dimethyl- a- (4- methyl- 3- pentenyl)cyclohex- 3- ene- 1- methanol |
| (R*,R*)-(1)-alpha,4-Dimethyl-alpha-(4-methyl-3-pentenyl)cyclohex-3-ene-1-methanol |
| (R*,R*)-.alpha.,4-Dimethyl-.alpha.-(4-methyl-3-pentenyl)-3-cyclohexene-1-methanol |
| (R*,R*)-.alpha.,4-Dimethyl-.alpha.-(4-methyl-3-pentenyl)cyclohex-3-ene-1-methanol |
| 3-Cyclohexene-1-methanol, alpha,4-dimethyl-alpha-(4-methyl-3-pentenyl)-, (alphaR,1R)-rel- |
| 3-Cyclohexene-1-methanol, alpha,4-dimethyl-alpha-(4-methyl-3-pentenyl)-, (R*,R*)- |
| CHEBI:192031 |
| RGZSQWQPBWRIAQ-LSDHHAIUSA-N |
| ALPHA-BISABOLOL, (+-)- |
| (R*,R*) - a,4 - dimethyl - a - (4 - methyl - 3 - pentenyl)cyclohex - 3 - ene - 1 - methanol |
| 3-ciclohexeno-1-metanol, Alfa,4-Dimetil-Alfa-(4-metil-3-penten-1-il)-, (Alfa R, 1R)-rel- |
| 3-Cyclohexene-1-methanol, .alpha.,4-dimethyl-.alpha.-(4-methyl-3-penten-1-yl)-, (.alpha.R,1R)-rel- |
| 3-Cyclohexene-1-methanol, alpha,4-dimethyl-alpha-(4-methyl-3-pentenyl)-, (theta,theta)-(+/-)- |
| (+/-)-.ALPHA.-BISABOLOL |
| .ALPHA.-BISABOLOL, (+)- |
| Tox21_111490 |
| BDBM50284328 |
| s6136 |
| AKOS015969722 |
| Tox21_111490_1 |
| .ALPHA.-BISABOLOL, (+/-)- |
| NCGC00095252-05 |
| ( inverted exclamation markA)-|A-Bisabolol |
| .ALPHA.-BISABOLOL (+)-FORM [MI] |
| HY-121222 |
| (+)-(1'R,2R)-.ALPHA.-BISABOLOL |
| CS-0081254 |
| W-105877 |
| (2R)-6-Methyl-2-[(1R)-4-methyl-3-cyclohexen-1-yl]-5-hepten-2-ol |
| (R*,R*)-(+-)-alpha,4-dimethyl-alpha-(4-methyl-3-pentenyl)cyclohex-3-ene-1-methanol |
| alpha,4-Dimethyl-alpha-(4-methyl-3-penten-1-yl)-(alphaR,1R)-3-Cyclohexene-1-methanol |
| 3-CYCLOHEXENE-1-METHANOL, .ALPHA.,4-DIMETHYL-.ALPHA.-(4-METHYL- 3-PENTEN-1-YL)-, (.ALPHA.R,1R)-REL- |
| 3-CYCLOHEXENE-1-METHANOL, .ALPHA.,4-DIMETHYL-.ALPHA.-(4-METHYL- 3-PENTENYL)-, (.THETA.,.THETA.)-(+/-)- |
| 3-CYCLOHEXENE-1-METHANOL, .ALPHA.,4-DIMETHYL-.ALPHA.-(4-METHYL- 3-PENTENYL)-, (R*,R*)- |
| 3-CYCLOHEXENE-1-METHANOL, .ALPHA.,4-DIMETHYL-.ALPHA.-(4-METHYL-3-PENTEN-1-YL)-, (.ALPHA.R,1R)- |
| 3-Cyclohexene-1-methanol, .alpha.,4-dimethyl-.alpha.-(4-methyl-3-pentenyl)-, [R-(R*,R*)]- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|