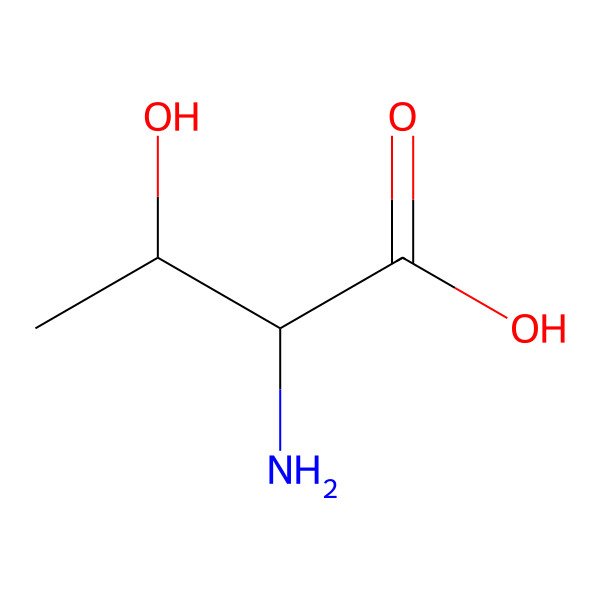
| threonine |
| 72-19-5 |
| Threonin |
| (2S,3R)-2-amino-3-hydroxybutanoic acid |
| L-(-)-Threonine |
| (S)-Threonine |
| H-Thr-OH |
| DL-Threonine |
| 2-amino-3-hydroxybutyric acid |
| Threonine, L- |
| Threonine (VAN) |
| 80-68-2 |
| Threoninum [Latin] |
| Treonina [Spanish] |
| thre |
| Threoninum |
| Treonina |
| (2S,3R)-2-Amino-3-hydroxybutyric acid |
| Threonine [USAN:INN] |
| (2S)-threonine |
| (2S,3R)-(-)-Threonine |
| L-Threonin |
| L-thr |
| EINECS 200-774-1 |
| Threonine, DL- |
| NSC 16589 |
| NSC 46701 |
| L-alpha-Amino-beta-hydroxybutyric acid |
| 2-Amino-3-hydroxybutanoic acid, (R-(R*,S*))- |
| DTXSID2046412 |
| FEMA NO. 4710 |
| UNII-2ZD004190S |
| CHEBI:16857 |
| Butanoic acid, 2-amino-3-hydroxy-, (R-(R*,S*))- |
| HSDB 7797 |
| L-2-Amino-3-hydroxybutyric acid |
| NSC-16589 |
| NSC-46701 |
| TFM6DU5S6A |
| 2ZD004190S |
| DTXCID0026412 |
| [R-(R*,S*)]-2-Amino-3-hydroxybutanoic acid |
| Threoninum (Latin) |
| 6028-28-0 |
| THREONINE (II) |
| THREONINE [II] |
| THREONINE (L) |
| thr |
| (2S,3R)-rel-2-Amino-3-hydroxybutanoic acid |
| THREONINE (MART.) |
| THREONINE [MART.] |
| (R-(R*,S*))-2-Amino-3-hydroxybutanoic acid |
| THREONINE (EP MONOGRAPH) |
| THREONINE [EP MONOGRAPH] |
| Threonine # |
| THREONINE (USP MONOGRAPH) |
| THREONINE [USP MONOGRAPH] |
| Allothreonine, D- |
| CAS-72-19-5 |
| (R-(R*,S*))-2-Amino-3-hydroxybutanoate |
| [R-(R*,S*)]-2-Amino-3-hydroxybutanoate |
| L-(U-14C)Threonine |
| MFCD00064270 |
| NSC46701 |
| beta-methylserine |
| L-Threonine; |
| NSC-206292 |
| NCGC00164520-01 |
| NCGC00164520-02 |
| Threonine (USP) |
| L-Threonine,(S) |
| H-Thr |
| (+/-)-threonine |
| L-Thr-OH |
| L-Threonine (9CI) |
| Threonine, labeled with carbon-14, L- |
| THREONINE [INN] |
| L-Threonine (JP17) |
| THREONINE [MI] |
| Threonine (L-Threonine) |
| THREONINE [HSDB] |
| THREONINE [INCI] |
| THREONINE [USAN] |
| UNII-TFM6DU5S6A |
| THREONINE [VANDF] |
| Threonine, L- (8CI) |
| 2-Amino-3-hydroxybutyrate |
| bmse000049 |
| bmse000810 |
| bmse000859 |
| L-THREONINE [FCC] |
| L-THREONINE [JAN] |
| L-Threonine (H-Thr-OH) |
| 2-Amino-3-hydroxybutanoate |
| SCHEMBL1480 |
| THREONINE [WHO-DD] |
| L-2-Amino-3-hydroxybutyrate |
| L-THREONINE [USP-RS] |
| L-Threonine non-animal source |
| CCRIS 8603 |
| CHEMBL291747 |
| GTPL4785 |
| CHEBI:26986 |
| DTXSID70893087 |
| L-alpha-Amino-beta-hydroxybutyrate |
| Pharmakon1600-01301008 |
| HY-N0658 |
| EINECS 201-300-6 |
| Tox21_112154 |
| AC7878 |
| NSC760118 |
| s4951 |
| (2S,3R)-2-Amino-3-hydroxybutyrate |
| AKOS006240505 |
| AKOS015840277 |
| Tox21_112154_1 |
| CCG-214540 |
| CS-W020046 |
| DB00156 |
| NSC 206292 |
| NSC-760118 |
| 7013-32-3 |
| AC-11296 |
| AS-12789 |
| L-Threonine, BioXtra, >=99.5% (NT) |
| L-Threonine, p.a., 99.0-101.0% |
| AI3-18477 |
| (2S,3R)-rel-2-Amino-3-hydroxybutanoicacid |
| [R-(R*,S*)]-2-amino-3-hydroxy-Butanoate |
| AM20100684 |
| T0230 |
| EN300-54609 |
| L-Threonine, reagent grade, >=98% (HPLC) |
| C00188 |
| D00041 |
| M02962 |
| [R-(R*,S*)]-2-amino-3-hydroxy-Butanoic acid |
| L-Threonine, Vetec(TM) reagent grade, >=98% |
| Q186521 |
| 3DD2E9AD-DB9A-460E-8A6B-C01B0F67AC4E |
| Q-201331 |
| LYSINE HYDROCHLORIDE IMPURITY C [EP IMPURITY] |
| 48: PN: WO2004076659 FIGURE: 7 claimed sequence |
| F8881-8883 |
| L-Threonine, certified reference material, TraceCERT(R) |
| Z813019058 |
| L-Threonine, European Pharmacopoeia (EP) Reference Standard |
| L-Threonine, United States Pharmacopeia (USP) Reference Standard |
| L-Threonine, Pharmaceutical Secondary Standard; Certified Reference Material |
| L-Threonine, from non-animal source, meets EP, JP, USP testing specifications, suitable for cell culture, 99.0-101.0% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|