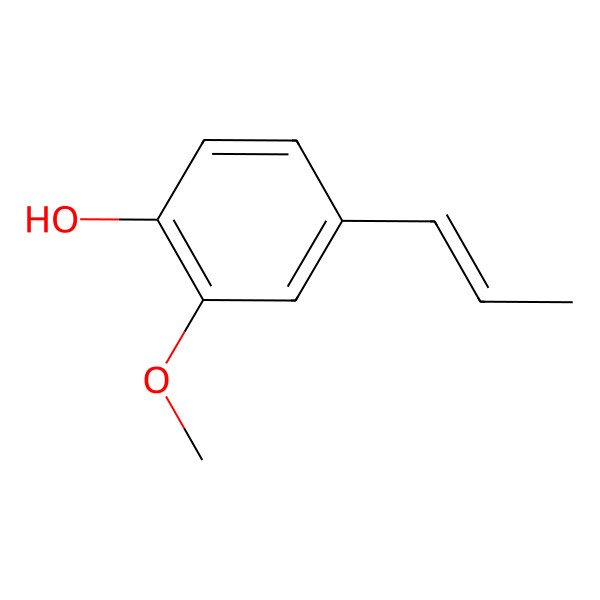
| 97-54-1 |
| (E)-Isoeugenol |
| trans-Isoeugenol |
| 2-Methoxy-4-propenylphenol |
| 4-Propenylguaiacol |
| 2-methoxy-4-(prop-1-en-1-yl)phenol |
| 5932-68-3 |
| 4-Hydroxy-3-methoxy-1-propenylbenzene |
| Isoeugenol trans-form |
| trans-p-Propenylquaiacol |
| 4-Hydroxy-3-methoxypropenylbenzene |
| 2-METHOXY-4-(1-PROPENYL)PHENOL |
| Phenol, 2-methoxy-4-propenyl- |
| 1-Hydroxy-2-methoxy-4-propenylbenzene |
| trans-2-Methoxy-4-propenylphenol |
| Propenylguaiacol |
| Isoeugenol (I) |
| Phenol, 2-methoxy-4-(1-propenyl)- |
| 2-methoxy-4-[(1E)-prop-1-en-1-yl]phenol |
| (E)-2-Methoxy-4-(prop-1-enyl)phenol |
| 1-Hydroxy-2-methoxy-4-propen-1-ylbenzene |
| Propenylgualacol |
| Iso-eugenol |
| 2-methoxy-4-[(E)-prop-1-enyl]phenol |
| NCI-C60979 |
| 3-Methoxy-4-hydroxypropenylbenzene |
| 3-Methoxy-4-hydroxy-1-propen-1-ylbenzene |
| NSC 6769 |
| CCRIS 744 |
| FEMA No. 2468 |
| Phenol, 2-methoxy-4-propenyl-, (E)- |
| Isoeugenol and isomers |
| CHEBI:50545 |
| 1-(3-Methoxy-4-hydroxyphenyl)-1-propene |
| 4-Hydroxy-3-methoxy-1-propen-1-ylbenzene |
| (E)-2-Methoxy-4-(prop-1-en-1-yl)phenol |
| Phenol, 2-methoxy-4-(1-propenyl)-, (E)- |
| trans-4-Propenylgualacol |
| EINECS 202-590-7 |
| EINECS 227-678-2 |
| UNII-28FSR1NAY4 |
| NSC 209522 |
| BRN 1909602 |
| BRN 2046156 |
| 28FSR1NAY4 |
| UNII-5M0MWY797U |
| 1-(3-Methoxy-4-hydroxyphenyl)-1-propane |
| AI3-15356 |
| CHEMBL193598 |
| Isoeugenol,c&t |
| HSDB 8044 |
| NSC-209522 |
| 2-Methoxy-4-[(1E)-1-propenyl]phenol |
| NCGC00091470-02 |
| Phenol, 2-methoxy-4-(1-propen-1-yl)- |
| Isoeugenol,predominantly trans |
| 3-06-00-04993 (Beilstein Handbook Reference) |
| DTXCID502413 |
| 2-Methoxy-4-[1-propenyl]phenol |
| UIL135434 |
| 3-Methoxy-4-hydroxy-1-propenylbenzene |
| CAS-97-54-1 |
| 2-methoxy-4-propenyl-Phenol |
| 4-06-00-06324 (Beilstein Handbook Reference) |
| DTXSID7022413 |
| isougenol |
| isoeugenol fcc |
| isoeugenol tech |
| e-isoeugenol |
| isoeugenol- |
| Iso eugenol |
| Isoeugenol E |
| Isoeugenol Z |
| iso-Eugenol 2 |
| isoeugenol 916 |
| CHISOEUG |
| iso eugenol 88%+ |
| isoeugenol extra nat us |
| Phenol, (E)- |
| Phenol, 2-methoxy-4-(1E)-1-propenyl- |
| ISOEUGENOL, E- |
| Isoeugenol, cis + trans |
| ISOEUGENOL, TRANS- |
| bmse010052 |
| ISOEUGENOL, (E)- |
| IE TRANS 92 |
| SCHEMBL57986 |
| MLS001065576 |
| BIDD:ER0697 |
| WLN: 2U1R DQ CO1 |
| Isoeugenol, analytical standard |
| Isoeugenol, predominantly trans |
| 5M0MWY797U |
| (E)-4-PROPENYLGUAIACOL |
| Isoeugenol, natural, 99%, FG |
| DTXSID50872350 |
| NSC6769 |
| HMS2268O09 |
| WLN: 2U1R DQ CO1 -E |
| (E)-2-methoxy-4-propenyl-Phenol |
| 2-methoxy-4-(1-propenyl)-Phenol |
| ISOEUGENOL TRANS-FORM [MI] |
| HY-N1952 |
| NSC-6769 |
| Tox21_111138 |
| Tox21_202433 |
| Tox21_300303 |
| BDBM50548724 |
| MFCD00009285 |
| NSC209522 |
| s5757 |
| STL264281 |
| 2-Methoxy-4-(1-propen-1-yl)phenol |
| AKOS000120575 |
| Tox21_111138_1 |
| DB14188 |
| LS-2028 |
| trans-2-methoxy-4-(1-propenyl)phenol |
| (E)-2-methoxy-4-(1-propenyl)-Phenol |
| 2-Methoxy-4-propenylphenol (isoeugenol) |
| Fenol, 2-metoxi-4-(1-propen-1-il)- |
| NCGC00091470-01 |
| NCGC00091470-03 |
| NCGC00091470-04 |
| NCGC00091470-05 |
| NCGC00254142-01 |
| NCGC00259982-01 |
| (E)-2-methoxy-4- (1-propenyl)-Phenol |
| AC-34996 |
| AS-61702 |
| SMR000112408 |
| (E)-2-Methoxy-4-(1-propen-1-yl)phenol |
| LS-104801 |
| CS-0018270 |
| I0132 |
| (E)-2-METHOXY-4-(1-PROPENYL)PHENOL |
| EN300-18429 |
| 2-methoxy-4-(1-propenyl)phenol (ACD/Name 4.0) |
| Isoeugenol, predominantly trans, analytical standard |
| Q27889971 |
| Z57953030 |
| (E) - 2 - methoxy - 4 - (prop - 1 - enyl)phenol |
| F1905-7039 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|