| 10191-41-0 |
| alpha-tochopherol |
| Ephanyl |
| Tocopheroxy radical |
| TOCOPHEROL |
| alpha-Tocopherol, DL- |
| all-rac-alpha-Tocopherol |
| 113085-06-6 |
| Tocopheroxyl radical |
| DL-alpha tocopherol |
| Vitamin E DL-alpha |
| dl-.alpha.-Tocopherol |
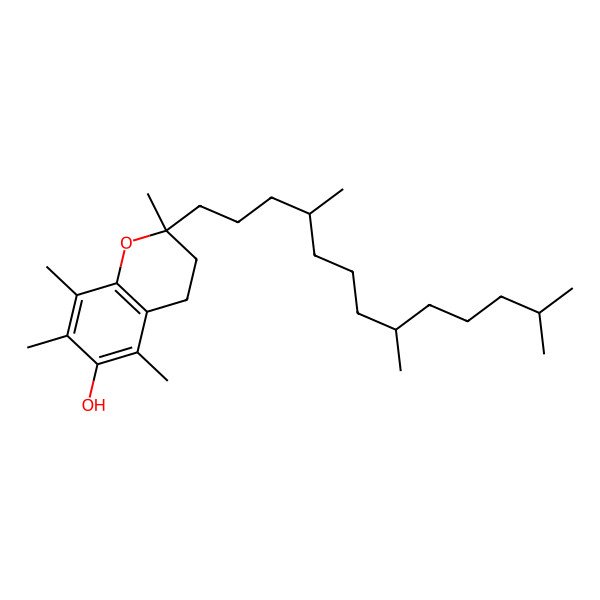
| 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydrochromen-6-ol |
| UNII-7QWA1RIO01 |
| DL-5,7,8-trimethyltocol |
| 7QWA1RIO01 |
| CHEMBL49563 |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)- |
| 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)chroman-6-ol |
| DL-alpha-Tocopherol (Vitamin E) |
| 1406-18-4 |
| 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydro-2H-1-benzopyran-6-ol |
| all-rac-alpha-Tocopherol (Vitamin E) |
| (2S, 4'R, 8'S)-alpha-Tocopherol |
| (2S, 4'S, 8'S)-alpha-Tocopherol |
| d-.alpha.-Tocopherol |
| (+-)-alpha-Tocopherol |
| 6-Chromanol, 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)- |
| alpha-Tocopherol-[D6] (Vitamin E-[D6]) |
| EC 233-466-0 |
| (2R)-alpha-Tocopherol (Mixture of Diastereomers) |
| Evitaminum |
| Almefrol |
| Emipherol |
| Etamican |
| Vitayonon |
| 5-17-04-00168 (Beilstein Handbook Reference) |
| Ilitia |
| CCRIS 5853 |
| Vitaplex E |
| Aquasol E |
| Covi-ox |
| Spavit E |
| Endo E |
| Vita E |
| .alpha.-Tocopherol |
| EINECS 233-466-0 |
| DL-Tocopherol |
| RRR-alpha-tocopherol |
| (2R, 4'S, 8'R)-alpha-Tocopherol |
| (2R, 4'S, 8'S)-alpha-Tocopherol |
| (2S, 4'R, 8'R)-alpha-Tocopherol |
| (2S, 4'S, 8'R)-alpha-Tocopherol |
| 2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydro-2H-chromen-6-ol |
| BRN 0094012 |
| (+/-)-alpha-Tocopherol |
| .Alpha.-tocopherol, DL- |
| 3,4-Dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-ol |
| SR-05000001813 |
| 5,6 Trans-Calcipotriol |
| DTXSID8021355 |
| 2,5,7,8-Tetramethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol |
| C29H50O2 |
| MFCD00072045 |
| NSC-20812 |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl- 2-(4,8,12-trimethyltridecyl)- |
| .alpha.-Tokoferol |
| .alpha. Tocopherol |
| 5,8-Trimethyltocol |
| Tocopherol (JP17) |
| Spectrum_001195 |
| DL- alpha -Tocopherol |
| DL-all-rac-A-Tocopherol |
| Spectrum2_000722 |
| Spectrum3_001338 |
| Spectrum4_001771 |
| Spectrum5_000381 |
| RONOTEC DF 120 |
| IRGANOX E 210 |
| UVINUL 2000AO |
| TOCOPHEROL, DL-ALPHA |
| SCHEMBL22303 |
| BSPBio_003095 |
| KBioGR_002282 |
| KBioSS_001675 |
| SPECTRUM310039 |
| (all-R)-.alpha.-Tocopherol |
| DivK1c_000533 |
| INS NO.307C |
| SPBio_000644 |
| RAC-.ALPHA.-TOCOPHEROL |
| alpha-Tocopherol, >=95.5% |
| INS-307C |
| SCHEMBL14315133 |
| (.+-.)-Med-E |
| (.+/-.)-.alpha.-Tocopherol |
| CHEBI:93909 |
| HMS501K15 |
| HSDB 8261 |
| KBio1_000533 |
| KBio2_001675 |
| KBio2_004243 |
| KBio2_006811 |
| KBio3_002315 |
| (2R,8'R)-.alpha.-Tocopherol |
| NINDS_000533 |
| HMS1923I03 |
| HMS2091C11 |
| Pharmakon1600-00310039 |
| 3, 4- dihydro- 2, 5, 7, 8- tetramethyl- 2- (4, 8, 12- trimethyltridecyl)- 2H- benzopyran- 6- ol |
| C29H50O2 (DL-alpha-tocopherol) |
| DL-ALPHA-TOCOPHEROL(307C) |
| NSC20812 |
| NSC82623 |
| TOCOPHEROL,DL-ALPHA [VANDF] |
| DL-.ALPHA.-TOCOPHEROL [MI] |
| DL-ALPHA TOCOPHEROL [MART.] |
| BBL027614 |
| BDBM50436220 |
| CCG-40162 |
| E-307C |
| MFCD00072051 |
| NSC-82623 |
| NSC755839 |
| s6104 |
| STL372809 |
| DL-ALPHA TOCOPHEROL [WHO-DD] |
| AKOS015960364 |
| ALL-RAC-ALPHA-TOCOPHEROL [FCC] |
| DB14476 |
| HY-W020044 |
| NSC-755839 |
| SDCCGMLS-0066634.P001 |
| .ALPHA.-TOCOPHEROL, DL- [II] |
| IDI1_000533 |
| NCGC00095254-01 |
| NCGC00095254-02 |
| NCGC00095254-03 |
| NCGC00095254-05 |
| NCGC00095254-06 |
| AC-10544 |
| J24.789H |
| SY012869 |
| SY057064 |
| VS-08569 |
| SBI-0051261.P003 |
| (+/-)-alpha-Tocopherol, analytical standard |
| CS-0031997 |
| FT-0600386 |
| FT-0624406 |
| FT-0625430 |
| FT-0696605 |
| T0251 |
| EN300-20897 |
| D02332 |
| D70247 |
| AB00051898_02 |
| SR-05000001813-1 |
| SR-05000001813-2 |
| (+/-)-alpha-Tocopherol, synthetic, >=96% (HPLC) |
| (+/-)-alpha-Tocopherol, tested according to Ph.Eur. |
| BRD-A82892199-001-01-7 |
| Q27165663 |
| (+/-)-alpha-Tocopherol, SAJ special grade, >=96.0% |
| F0001-2420 |
| 7A4513C3-131C-41D4-B6A0-7ED994AC2947 |
| all-rac-alpha-TOCOPHERYL ACETATE IMPURITY C [EP IMPURITY] |
| alpha-Tocopherol, British Pharmacopoeia (BP) Reference Standard |
| DL-alpha-Tocopherol (Vitamin E) 10 microg/mL in Acetonitrile |
| 2,5,7,8-Tetramethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol # |
| alpha-Tocopherol, European Pharmacopoeia (EP) Reference Standard |
| Alpha Tocopherol, United States Pharmacopeia (USP) Reference Standard |
| Alpha Tocopherol, Pharmaceutical Secondary Standard; Certified Reference Material |
| 16826-11-2 |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, radical ion(1+), (2R-(2R*(4R*,8R*)))- |
| 2H-1-Benzopyran-6-ol,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, [2R*(4R*,8R*)]-(.+-.)- |
| 2H-1-Benzopyran-6-ol,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, [2R-[2R*(4R*,8R*)]]- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|