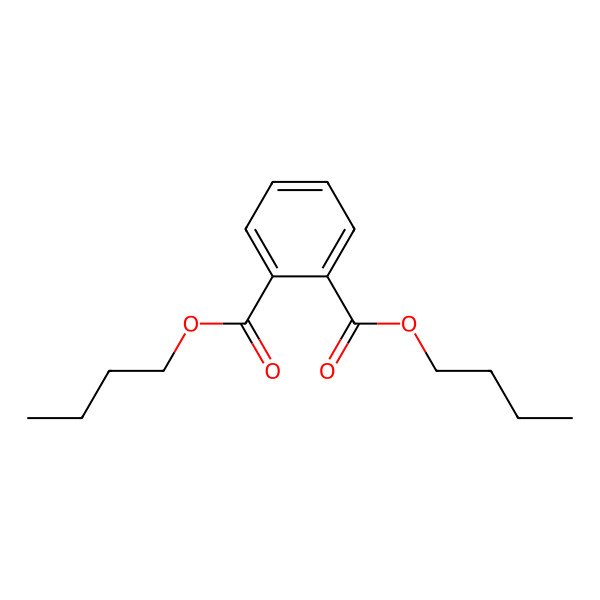
| 84-74-2 |
| Di-n-butyl phthalate |
| n-Butyl phthalate |
| Butyl phthalate |
| Genoplast B |
| Palatinol C |
| Celluflex DPB |
| DIBUTYLPHTHALATE |
| Unimoll DB |
| Staflex DBP |
| Elaol |
| Ergoplast FDB |
| Witcizer 300 |
| Kodaflex DBP |
| Hexaplas M/B |
| Dibutyl-o-phthalate |
| Dibutyl 1,2-benzenedicarboxylate |
| Phthalic acid dibutyl ester |
| dibutyl benzene-1,2-dicarboxylate |
| 1,2-Benzenedicarboxylic acid, dibutyl ester |
| DBP (ester) |
| N-Butylphthalate |
| Ersoplast FDA |
| Uniflex DBP |
| Hatcol DBP |
| Phthalate, di-n-butyl |
| Dibutyl o-phthalate |
| RC Plasticizer DBP |
| dibutyl-phthalate |
| Phthalic acid, dibutyl ester |
| Benzene-o-dicarboxylic acid di-n-butyl ester |
| o-Benzenedicarboxylic acid, dibutyl ester |
| Phthalate, dibutyl- |
| RCRA waste number U069 |
| nutyl phthalate |
| Caswell No. 292 |
| Monocizer dbp |
| Plasthall dbp |
| Vestinol c |
| Hatco dbp |
| Phthalate, Butyl |
| PX 104 |
| Phthalic acid di-n-butyl ester |
| Phthalate, Dibutyl |
| Ruifeng vp 201 |
| Corflex 440 |
| NSC 6370 |
| Di n Butyl Phthalate |
| ortho-Dibutyl phthalate |
| Yh-1bd2 |
| CCRIS 2676 |
| HSDB 922 |
| Di-n-butylorthophthalate |
| Di-n-butylester kyseliny ftalove |
| DTXSID2021781 |
| CHEBI:34687 |
| Dibutyl-1,2-benzenedicarboxylate |
| Dibutylphthatlate |
| dibutyl phthalate (DBP) |
| Palatinol DBP |
| NSC-6370 |
| EINECS 201-557-4 |
| di-n-butyl phthalate (DBuP) |
| EPA Pesticide Chemical Code 028001 |
| UNII-2286E5R2KE |
| BRN 1914064 |
| Morflex 240 |
| o-Benzenedicarboxylic acid dibutyl ester |
| Uniplex 150 |
| 1,2-Benzenedicarboxylic acid dibutyl ester |
| Vp-201 |
| 2286E5R2KE |
| AI-3-00283 |
| Dibutyl phthalate [NF] |
| Benzenedicarboxylic acid dibutyl ester |
| RCRA waste no. U069 |
| Di-n-butylester kyseliny ftalove [Czech] |
| CHEMBL272485 |
| DTXCID301781 |
| phthalate dibutyl |
| Benzene-o-dicarboxylic acid, di-n-butyl ester |
| NSC6370 |
| Dibutyl 1,2-benzene-dicarboxylate |
| EC 201-557-4 |
| 1,2-Benzenedicarboxylic acid, 1,2-dibutyl ester |
| 1,2-dibutyl benzene-1,2-dicarboxylate |
| Dibutyl ester of 1,2-benzenedicarboxylic acid |
| DIBUTYL PHTHALATE (II) |
| DIBUTYL PHTHALATE [II] |
| DIBUTYL PHTHALATE (MART.) |
| DIBUTYL PHTHALATE [MART.] |
| DIBUTYL PHTHALATE (USP-RS) |
| DIBUTYL PHTHALATE [USP-RS] |
| CAS-84-74-2 |
| DIBUTYL PHTHALATE (EP MONOGRAPH) |
| DIBUTYL PHTHALATE [EP MONOGRAPH] |
| RAPIDCELLtrade markP |
| SR-05000001549 |
| dibutylphthalat |
| Dibutylftalat |
| Bufa |
| dibutyl phtalate |
| di-butylphthalate |
| dibutly phthalate |
| Ergoplast FOB |
| Synolate MD |
| Bisoflex DBP |
| Induflex DBP |
| Mollan B |
| Dibutyll phthalate |
| Ersoplast FDA. |
| Hatcp DBP |
| MFCD00009441 |
| phthalsauredibutylester |
| Benzenedicarboxylic acid, dibutyl ester |
| Ftalato di n-butanolo |
| Bis-N-butyl phthalate |
| dibutyl phthalate; DBP |
| Sicol 140 |
| Spectrum_001975 |
| Di(1-butyl) phthalate |
| DPA (CHRIS Code) |
| Phthalsaeure dibutylester |
| SpecPlus_000628 |
| Dibutyl phthalate, 99% |
| Spectrum3_000874 |
| Spectrum4_000714 |
| Spectrum5_002068 |
| WITICIZER 300 |
| D07FLG |
| DBP (Dibutyl phthalate) |
| Epitope ID:138714 |
| WLN: 4OVR BVO2 |
| Di-n-butylphthalate (DBP) |
| di-n-butyl phthalate (dbp) |
| Dibutyl phthalate, >=99% |
| SCHEMBL24051 |
| BSPBio_002547 |
| KBioGR_001267 |
| KBioSS_002541 |
| R!C. PLASTICIZER DBP |
| SPECTRUM330086 |
| MLS002177802 |
| BIDD:ER0641 |
| DivK1c_006724 |
| DIBUTYL PHTHALATE [MI] |
| Phthalic acid, bis-butyl ester |
| GTPL6295 |
| Di-n-butyl l phthalate (BBP) |
| Di-n-butyl l phthalate (DBP) |
| DIBUTYL PHTHALATE [HSDB] |
| DIBUTYL PHTHALATE [INCI] |
| KBio1_001668 |
| KBio2_002532 |
| KBio2_005100 |
| KBio2_007668 |
| KBio3_002047 |
| BUTYL PHTHALATE [WHO-DD] |
| Dibutyl phthalate, AR, >=99% |
| Dibutyl phthalate, LR, >=98% |
| Dibutyl 1, 2-benzenedicarboxylate |
| Dibutyl-1,2-benzene-dicarboxylate |
| HMS2091E09 |
| HMS3041E18 |
| Pharmakon1600-00330086 |
| BCP24796 |
| HY-Y0304 |
| Benzol-1,2-dicarbonsauredibutylester |
| Dibutyl phthalate, Selectophore(TM) |
| Tox21_201729 |
| Tox21_300980 |
| BBL011532 |
| BDBM50371946 |
| NA9095 |
| NSC755894 |
| STL146650 |
| AKOS005720807 |
| Di(n-butyl) 1,2-benzenedicarboxylate |
| CCG-230933 |
| DB13716 |
| LS-1840 |
| NSC-755894 |
| USEPA/OPP Pesticide Code: 028001 |
| NCGC00090769-01 |
| NCGC00090769-02 |
| NCGC00090769-03 |
| NCGC00090769-04 |
| NCGC00090769-05 |
| NCGC00090769-06 |
| NCGC00090769-07 |
| NCGC00090769-08 |
| NCGC00090769-09 |
| NCGC00254882-01 |
| NCGC00259278-01 |
| SMR000777923 |
| SBI-0052568.P002 |
| CS-0013564 |
| Dibutyl phthalate, ReagentPlus(R), >=99% |
| FT-0624680 |
| P0292 |
| S5377 |
| EN300-77394 |
| 1,2 Benzenedicarboxylic acid, di-n-butyl ester |
| 1,2-Benzenedicarboxylic acid 1,2-dibutyl ester |
| Dibutyl phthalate, SAJ special grade, >=98.0% |
| 1,2 Benzenedicarboxylic acid, bis(n-butyl) ester |
| Q415612 |
| J-503795 |
| SR-05000001549-1 |
| SR-05000001549-3 |
| BRD-K73477617-001-01-0 |
| BRD-K73477617-001-04-4 |
| Dibutyl phthalate, PESTANAL(R), analytical standard |
| F0001-2134 |
| Z277540112 |
| Dibutyl phthalate, certified reference material, TraceCERT(R) |
| Dibutyl phthalate, European Pharmacopoeia (EP) Reference Standard |
| DIBUTYL PHTHALATE (SEE ALSO PEROXISOME PROJECT (DIBUTYL PHTHALATE)) |
| Dibutyl phthalate, United States Pharmacopeia (USP) Reference Standard |
| PEROXISOME PROJECT (DIBUTYL PHTHALATE) (SEE ALSO DIBUTYL PHTHALATE) |
| Dibutyl Phthalate, Pharmaceutical Secondary Standard; Certified Reference Material |
|
There are more than 10 synonyms. If you wish to see them all click here.
|