| 65-46-3 |
| Cytosine riboside |
| 1-beta-D-Ribofuranosylcytosine |
| 4-Amino-1-beta-D-ribofuranosyl-2(1H)-pyrimidinone |
| 1beta-Ribofuranosylcytosine |
| Cytidin |
| beta-D-Ribofuranoside, cytosine-1 |
| 1-beta-Ribofuranosylcytosine |
| 1beta-D-Ribofuranosylcytosine |
| 4-Amino-1beta-D-ribofuranosyl-2(1H)-pyrimidinone |
| Zytidin |
| Cytosine, 1-beta-D-ribofuranosyl- |
| 1beta-2'-Ribofuranosylcytosine, d- |
| Cyd |
| NSC 20258 |
| cytosine-1beta-D-Ribofuranoside |
| CHEBI:17562 |
| 2(1H)-Pyrimidinone, 4-amino-1-beta-D-ribofuranosyl- |
| EINECS 200-610-9 |
| MFCD00006545 |
| UNII-5CSZ8459RP |
| 2(1H)-Pyrimidinone, 4-amino-1beta-D-ribofuranosyl- |
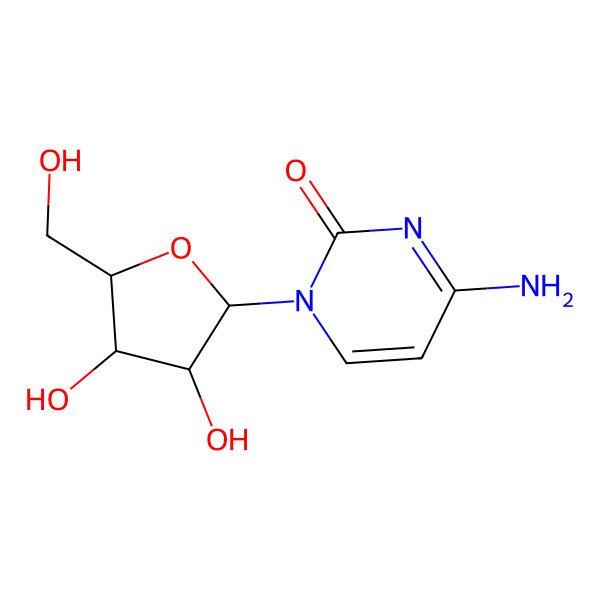
| 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one |
| 5CSZ8459RP |
| 4-amino-1-beta-D-ribofuranosylpyrimidin-2(1H)-one |
| NSC-20258 |
| 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one |
| 4-amino-1-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one |
| C9H13N3O5 |
| 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2-dihydropyrimidin-2-one |
| SMR000058243 |
| 1.beta.-Ribofuranosylcytosine |
| 1.beta.-D-Ribofuranosylcytosine |
| beta-cytidine |
| cytidin- |
| Cytidine- |
| .beta.-D-Ribofuranoside, cytosine-1 |
| 3h-cytidine |
| Cytosine, 1-.beta.-D-ribofuranosyl- |
| Posilent (TN) |
| CTN |
| beta.-D-Ribo-C |
| Cytidine, 99% |
| Cytosine b-D-riboside |
| CYTIDINE [INCI] |
| 1-b-D-ribosyl-Cytosine |
| CYTIDINE [MI] |
| Cytosine-beta-D-riboside |
| CP-C |
| 4-Amino-1.beta.-D-ribofuranosyl-2(1H)-pyrimidinone |
| CYTIDINE [MART.] |
| bmse000190 |
| bmse000969 |
| bmse001020 |
| CYTIDINE [WHO-DD] |
| D0E7ES |
| Epitope ID:141494 |
| 2(1H)-Pyrimidinone, 4-amino-1-.beta.-D-ribofuranosyl- |
| SCHEMBL7179 |
| 1-b-D-Ribofuranosylcytosine |
| cytosine-1b-D-Ribofuranoside |
| beta-D-ribofuranosyl-cytidine |
| MLS000049947 |
| MLS002207040 |
| 1-beta-D-ribosyl- (6CI) |
| 1-beta-delta-ribosyl-Cytosine |
| CHEMBL95606 |
| Cytosine-1-b-D-ribofuranoside |
| GTPL4728 |
| 1-beta-D-ribofuranosyl-Cytosine |
| cytosine-1b-delta-Ribofuranoside |
| SCHEMBL24781031 |
| 1beta-delta-Ribofuranosylcytosine |
| DTXSID60891552 |
| 1-beta-delta-Ribofuranosylcytosine |
| Cytidine, >=99.0% (HPLC) |
| cytosine-1beta-delta-Ribofuranoside |
| 1-beta-delta-ribofuranosyl-Cytosine |
| HY-B0158 |
| MMV638723 |
| s2053 |
| AKOS015888568 |
| AM83932 |
| CCG-266896 |
| CS-1989 |
| DB02097 |
| SRI-2352_17 |
| NCGC00093356-08 |
| NCGC00142483-01 |
| NCGC00142483-09 |
| AS-12696 |
| BP-58628 |
| LS-135845 |
| C-9850 |
| C00475 |
| D07769 |
| EN300-184125 |
| 1-(beta-D-ribofuranosyl)-4-aminopyrimidin-2-one |
| 4-Amino-1-b-D-ribofuranosyl-2(1H)-pyrimidinone |
| Cytosine -D-riboside;Cytosine-1--D-ribofuranoside |
| Q422538 |
| J-700167 |
| 4-Amino-1-beta-D-ribofuranosyl-2-(1H)-pyrimidinone |
| BRD-K71847383-001-12-5 |
| 4-Amino-1-.beta.-D-ribofuranosyl-2-(1H)-pyrimidinone |
| 4-Amino-1-beta-delta-ribofuranosyl-2(1H)-pyrimidinone |
| F0348-2240 |
| Z1879263740 |
| 1-(b-D-Ribofuranosyl)-2-oxo-4-amino-1,2-dihydro-1,3-diazine |
| 6D2DC474-DD76-4081-8B34-10605C218F49 |
| 1-(b-delta-Ribofuranosyl)-2-oxo-4-amino-1,2-dihydro-1,3-diazine |
| 1-(beta-D-Ribofuranosyl)-2-oxo-4-amino-1,2-dihydro-1,3-diazine |
| Cytidine, BioReagent, suitable for cell culture, powder, >=99% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|