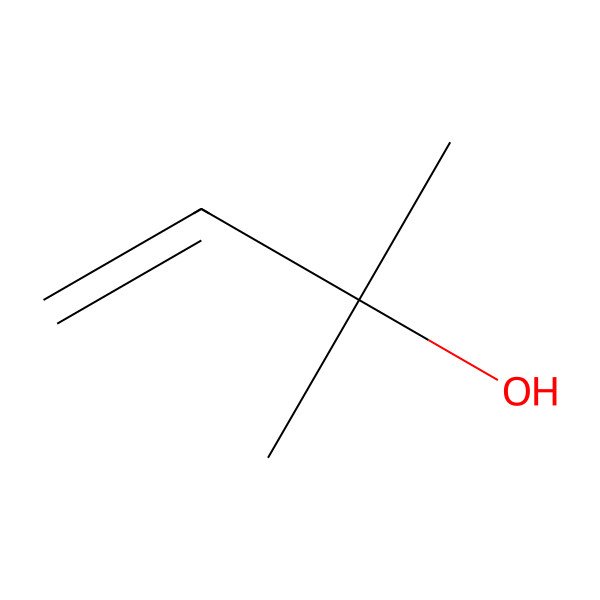
| 115-18-4 |
| 2-Methylbut-3-en-2-ol |
| Methylbutenol |
| 3-Buten-2-ol, 2-methyl- |
| 1,1-Dimethylallyl alcohol |
| 3-Hydroxy-3-methyl-1-butene |
| 3-Hydroxy-3-methylbutene |
| Dimethylvinylcarbinol |
| Dimethylvinylmethanol |
| Vinyldimethylcarbinol |
| isoprenyl alcohol |
| 3-Methyl-1-buten-3-ol |
| 1,1-Dimethyl-2-propenol |
| 2-Methyl-3-butene-2-ol |
| 2-Methyl-2-hydroxy-3-butene |
| 1,1-Dimethylallyl alchol |
| 2-Methyl-3-buten-2-yl alcohol |
| alpha,alpha-Dimethylallyl alcohol |
| 1,1-Dimethyl-2-propanol |
| 3-Methyl-buten-(1)-ol-(3) |
| CH2=CHC(CH3)2OH |
| NSC 15977 |
| 2-Methyl but-3-ene-2-ol |
| 1-Buten-3-ol, 3-methyl- |
| EINECS 204-068-4 |
| .alpha.,.alpha.-Dimethylallyl alcohol |
| 3-Methyl-1-butene-3-ol |
| 2-methyl-but-3-en-2-ol |
| BRN 1698263 |
| UNII-SH64HE46L9 |
| 3-Methyl-buten-(1)-ol-(3) [German] |
| 3-hydroxy-3-methylbut-1-ene |
| AI3-23122 |
| SH64HE46L9 |
| 1,1-Dimethyl-2-propen-1-ol |
| DTXSID3047471 |
| NSC-15977 |
| 3-Butyn-2-ol, 2-methyl- (8CI,9CI) |
| EC 204-068-4 |
| 4-01-00-02132 (Beilstein Handbook Reference) |
| dimethyl vinyl carbinol |
| MFCD00004470 |
| 2-vinyl-2-propanol |
| 3-methyl-buten-3-ol |
| 1-dimethyl-2-propenol |
| 1-dimethylallyl alcohol |
| MBL (CHRIS Code) |
| 3-methylbut-1-en-3-ol |
| 2-methylbut-3-ene-2-ol |
| 3-methyl-but-1-en-3-ol |
| 1, 1-Dimethyl-2-propenol |
| 1, 1-Dimethylallyl alcohol |
| 3-methyl-3-hydroxybut-1-ene |
| CHEMBL4245903 |
| DTXCID1027471 |
| 1,1-Dimethyl-2-propenyl alcoho |
| 1,1-dimethyl-2-propenyl alcohol |
| AMY3791 |
| CHEBI:132752 |
| 2-Methyl-3-buten-2-ol, 98% |
| NSC15977 |
| HYDROXY-3-METHYLBUTENE, 3- |
| Tox21_303823 |
| STL570064 |
| 2-Methyl-3-buten-2-ol, >=98% |
| AKOS009156785 |
| BUT-3-EN-2-OL, 2-METHYL- |
| SB83756 |
| NCGC00357265-01 |
| 2 - methylbut - 3 - en - 2 - ol |
| CAS-115-18-4 |
| LS-47240 |
| 2-Methyl-3-buten-2-ol, analytical standard |
| FT-0612937 |
| M0178 |
| EN300-84043 |
| C21402 |
| Q209432 |
| InChI=1/C5H10O/c1-4-5(2,3)6/h4,6H,1H2,2-3H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|